
Medicaid Coverage of
Medication-Assisted
Treatment for Alcohol
and Opioid Use
Disorders and of
Medication
for the Reversal of
Opioid Overdose

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE ii
Acknowledgments
This report was prepared for the Substance Abuse and Mental Health Services Administration
(SAMHSA) by IBM® Watson Health™ as subcontractor to the National Council for Behavioral
Health under Contract #HHSS283201200031I/HHSS28342002T with SAMHSA, U.S.
Department of Health and Human Services (HHS). Mitchell Berger served as the Government
Project Officer and Kaitlin Abell as the Contracting Officer Representative.
Disclaimer
The views, opinions, and content of this publication are those of the author and do not
necessarily reflect the views, opinions, or policies of SAMHSA or HHS. The listing of
nonfederal resource is not all inclusive, and inclusion on the listing does not constitute
endorsement by SAMHSA or HHS.
Public Domain Notice
All material appearing in this report is in the public domain and may be reproduced or copied
without permission from SAMHSA. Citation of the source is appreciated. However, this
publication may not be reproduced or distributed for a fee without the specific, written authority
of the Office of Communications, SAMHSA, HHS.
Electronic Access and Copies of Publication
This publication may be downloaded or ordered from http://www.oas.samhsa.gov. Or call
SAMHSA at 1-877-SAMHSA-7 (1-877-726-4727) (English and Español).
Recommended Citation
Substance Abuse and Mental Health Services Administration. Medicaid Coverage of
Medication-Assisted Treatment for Alcohol and Opioid Use Disorders and of Medication for the
Reversal of Opioid Overdose. HHS Publication No. SMA-18-5093. Rockville, MD: Substance
Abuse and Mental Health Services Administration, 2018.
Originating Office
Office of Policy, Planning and Innovation, Substance Abuse and Mental Health Services
Administration, 5600 Fishers Lane, Rockville, MD 20857. HHS Publication No. SMA-18-5093,
2018. HHS Publication No. SMA-18-5093. Published in 2018.
Nondiscrimination Notice
SAMHSA complies with applicable federal civil rights laws and does not discriminate on the
basis of race, color, national origin, age, disability, or sex. SAMHSA cumple con las leyes
federales de derechos civiles aplicables y no discrimina por motivos de raza, color, nacionalidad.
HHS Publication No. SMA-18-5093
Published in 2018
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE iii
Table of Contents
Table of Contents ........................................................................................................................... iii
Executive Summary ........................................................................................................................ 1
Background ................................................................................................................................. 1
Study Methods ............................................................................................................................ 1
Key Findings ............................................................................................................................... 2
State Medicaid Program Reimbursement for and Limitations on MAT and Naloxone ......... 2
Key Findings on Innovative Approaches to Financing and Delivering MAT ........................ 4
I. Introduction .......................................................................................................................... 5
II. State Considerations for Covering Medications for Alcohol and Opioid Use Disorders ... 7
A. Efficacy of Medications Used to Treat Alcohol and Opioid Use Disorders .................... 7
Medications for Alcohol Use Disorders ................................................................................. 8
Medications for Opioid Use Disorders ................................................................................. 10
Medications for Opioid Overdose ......................................................................................... 14
Cost Offset and Cost Effectiveness of Medications for Alcohol and Opioid Use Disorders ... 15
Alcohol Use Disorder Costs.................................................................................................. 15
Opioid Use Disorder Costs ................................................................................................... 16
State and Federal Regulations and Policies Affecting the Prescription and Dispensing of
Medications for Alcohol and Opioid Use Disorders................................................................. 18
Federal Laws Governing Methadone and Buprenorphine .................................................... 18
Requirements of Parity ......................................................................................................... 19
State Laws and Policies ........................................................................................................ 20
Professional Licensure and Certification .............................................................................. 20
Facility Licensing and Certification...................................................................................... 21
Billing Requirements ............................................................................................................ 21
Data Exchange ...................................................................................................................... 22
Other Policies That May Affect Delivery of MAT ............................................................... 22
III. Medicaid Coverage of Medications for Alcohol and Opioid Use Disorders ..................... 26
A. Data Sources and Methodology ..................................................................................... 26
Rules for Determining Coverage .......................................................................................... 26
Rules for Determining Preferred Status and Benefit Limitations ......................................... 27
Approach to Analysis of Methadone Coverage.................................................................... 29
Methodology Limitations...................................................................................................... 32
Medicaid Benefit Limits on Medications for Alcohol and Opioid Use Disorders ................... 32
IV. Innovative MAT Provision, Coverage, and Financing Models ......................................... 41
A. Innovative Models.......................................................................................................... 41
Nurse Care Management Model, Massachusetts .................................................................. 41
Statewide Integration of MAT into SUD Treatment, Missouri ............................................ 43
Flex Care Telemedicine-MAT Pilot Project and Other Innovative MAT Approaches,
Washington ........................................................................................................................... 45
B. Cross-Cutting Best Practices.......................................................................................... 47
V. Conclusion ......................................................................................................................... 50
A. Coverage of Medications Used for MAT....................................................................... 50
B. Benefit Design................................................................................................................ 50
C. Other Considerations...................................................................................................... 51
D. Lessons Learned from Innovative Approaches to Financing MAT. .............................. 51
VI. References .......................................................................................................................... 53
VII. Appendix A. Coverage of Medications for Alcohol and Opioid Use Disorders by State . 69
VIII. Appendix B. Authors and Acknowledgements ................................................................ 113
Authors ................................................................................................................................ 113
Acknowledgements ............................................................................................................. 113
Tables
Table 1. Medications Used to Treat Alcohol Use Disorders .......................................................... 8
Table 2. Medications Used to Treat Opioid Use Disorders .......................................................... 11
Table 3. Federal Prescribing Regulations for Medications Used to Treat Alcohol and Opioid Use
Disorders or to Reverse Opioid Overdose .................................................................................... 19
Figures
Figure 1. Medicaid Coverage of Medications for Alcohol and Opioid Use Disorders or to
Reverse Opioid Overdose, 2018
a
.................................................................................................. 34
Figure 2. Preferred Status of Medications for Alcohol and Opioid Use Disorders or to Reverse
Opioid Overdose, 2018
a
................................................................................................................ 35
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE iv
Figure 3. Prior Authorization Requirements for Medications for Alcohol and Opioid Use
Disorders or to Reverse Opioid Overdose, 2018
a
......................................................................... 36
Figure 4. Prior Authorization Requirements for Extended-Release Injectable Naltrexone, 2018
a
Figure 5. Quantity or Dosing Limit Requirements for Medications for Alcohol and Opioid Use
....................................................................................................................................................... 37
Disorders or to Reverse Opioid Overdose, 2018
a
......................................................................... 38
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE v

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
1
Executive Summary
This report presents summary information on Medicaid coverage and financing of medications to
treat alcohol and opioid use disorders. Medicaid serves over 72 million adult and child
beneficiaries annually (Centers for Medicare & Medicaid Services [CMS], 2017a). An estimated
12 percent of adults over 18 years of age and 6 percent of adolescents aged 12 to 17 years in
Medicaid have a substance use disorder (SUD)
1
(CMS, 2014, 2015). Given the important role
that medications can play in treating these disorders, it is critical that Medicaid programs develop
clinically effective and cost-effective delivery and financing approaches to providing these
medications to beneficiaries. The present report is intended to serve as a resource guide for those
efforts.
Background
Excessive alcohol consumption and/or the use of illicit drugs have been clearly linked to adverse
health and social outcomes (Bouchery, Harwood, Sacks, Simon, & Brewer, 2011; Devlin &
Henry, 2008; National Institute on Drug Abuse, 2012a). There are an estimated 88,000 deaths
each year due to the use of alcohol (Centers for Disease Control and Prevention, 2013), and drug
overdose is the leading cause of accidental death in the United States, with more than 72,000
lethal drug overdoses estimated in 2017 and 63,632 in 2016 (National Institute on Drug Abuse,
2018). Of the 63,632 drug overdoses in 2016, 42,249 deaths involved an opioid and, out of those,
15,469 involved heroin (Seth, Scholl, Rudd, & Bacon, CDC MMWR, 2018).
Fortunately, the U.S. Food and Drug Administration (FDA) has approved effective medications
for treating alcohol and opioid use disorders. These medications include:
• disulfiram and acamprosate for treatment of alcohol dependence;
• methadone, buprenorphine (including oral, subdermal and injectable extended-release
formulations), and buprenorphine-naloxone (including oral, buccal, and sublingual) for
treatment of opioid dependence; and
• naltrexone (oral and an injectable extended-release formulation) for treatment of alcohol
or opioid dependence.
In addition, naloxone is an effective medication used to reverse opioid overdose.
Study Methods
The research team retrieved the most recent pharmacy and behavioral health documents from
state Medicaid agency websites during the second and third quarters of 2018. Searches were
conducted for information related to Medicaid pharmaceutical coverage of and benefit design for
medication-assisted treatment (MAT), including selected Medicaid managed care plans, if
1
According to the Diagnostic and Statistical Manual of Mental Disorders, 5
th
ed. (DSM-5), an SUD is characterized
by a problematic pattern of using a substance that results in impairment in daily life or noticeable stress.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
2
available. Internet queries sought documents providing information on formularies, Preferred
Drug Lists (PDLs), prior authorization, psychosocial treatment requirements, quantity limits, and
other pharmacy benefit limitations. Where ambiguity remained, the research team accessed
online drug search tools such as Epocrates® (https://online.epocrates.com/rxmain), which
provided additional information that allowed us to pursue the subject further using other
documents. Medications may be covered by state Medicaid programs without being listed on an
easily accessible formulary; therefore, when no other evidence of coverage was found in the
Medicaid or other documents, a final determination of whether Medicaid coverage existed was
made by searching the 2018 Medicaid State Drug Utilization Data (CMS, n.d.).
Key Findings
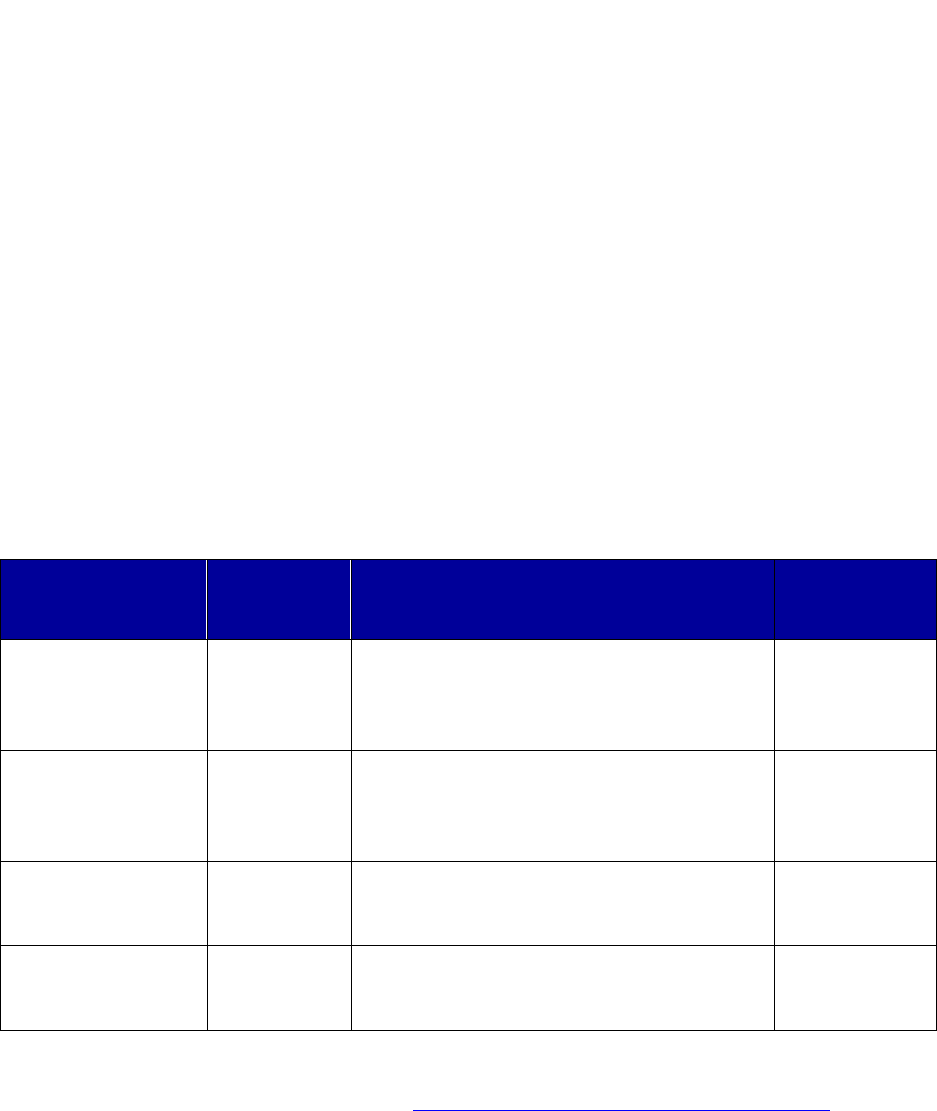
State Medicaid Program Reimbursement for and Limitations on MAT and Naloxone
All states reimburse for some form of medications for MAT. A review of Medicaid policies
and data revealed that all states reimburse some form of buprenorphine, buprenorphine-
naloxone, oral naltrexone, and extended-release naltrexone and that most states cover disulfiram
and acamprosate. All states also reimburse for some form of naloxone, the opioid overdose
reversal medication. Only 42 state Medicaid programs, however, reimburse for methadone as
MAT (in contrast to reimbursing for the use of methadone to treat pain and other conditions),
and fewer than 70 percent of states reimburse for implanted or extended-release injectable
buprenorphine.
Even if state Medicaid agencies reimburse for specific medications, they may impose
certain constraints, or benefit design limits, on obtaining the medication. State Medicaid
agencies typically rely on a Pharmacy and Therapeutics Committee or equivalent entity to
determine whether to reimburse for a medication and whether it will be assigned preferred or
nonpreferred status, a subject addressed in greater detail below and in Section I of this report.
These committees establish PDLs, which typically list the first-choice medication(s) preferred
for a given condition for Medicaid patients. State Medicaid agencies also may establish other
benefit design limits that must be satisfied in order to obtain reimbursement for the medication.
These limits may include quantity or dosing limits, prior authorization, requirements for
psychosocial treatment, and step therapy, the latter three of which are known as “nonquantitative
treatment limitations,” or NQTLs. Each of these benefit design features are addressed in greater
detail below and in Section I of this report.
Reimbursement of medications as MAT does not mean that they all have preferred status
within state Medicaid programs. Reimbursement may be available for a medication even if
the medication does not have preferred status; however, if a medication does not have preferred
status, the prescriber usually must obtain permission from the member’s pharmacy benefit plan

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
3
before the product can
be reimbursed. In some
instances, a drug still
may require
authorization even if it
has a preferred status.
Preferred status of a
drug also can vary
between fee-for-service
and managed care
plans within a state’s
Medicaid program.
The majority of state
Medicaid programs
assign preferred status
to all of the MAT
medications and
naloxone, with the
exceptions of the extended-release versions of buprenorphine. In addition, we concluded that at
least one formulation of naloxone, which is used to reverse opioid overdose, was covered with
preferred status in 43 state Medicaid programs.
State Medicaid programs routinely use pharmacy benefit management requirements, such
as prior authorization, to contain expenditures and encourage the proper use of
medications, including for the treatment of alcohol and opioid disorders. Research on the
use of prior authorization requirements with psychiatric medications has revealed that prior
authorization can reduce medication expenditures. However, these requirements also can have
the unintended consequence of preventing timely access to treatment. Potential barriers to access
may be reduced as payers and providers move from a paper-based prior authorization process to
an electronic, real-time, standardized process. An electronic process may integrate better into
providers’ workflow and may reduce the time that providers and patients must wait to secure
authorization. Among the medications reviewed, prior authorization was required most
commonly for buprenorphine and buprenorphine-naloxone, in 40 and 31 states, respectively.
Most prior authorization requirements for buprenorphine monotherapy exist because of its
potential for abuse, and many include restrictions limiting it to use by pregnant women and in
other limited applications. Prior authorization for extended-release injectable naltrexone is
required in 19 states, likely because it is not available in generic form, making it more expensive,
and because patients must abstain from opioids for a minimum of 7 days prior to receiving the
injection. Extended-release forms of buprenorphine also require prior authorization in
approximately half of all states; they also are brand drugs and require that the patient be
stabilized on other medication before their use. Relatively few states require authorization for the
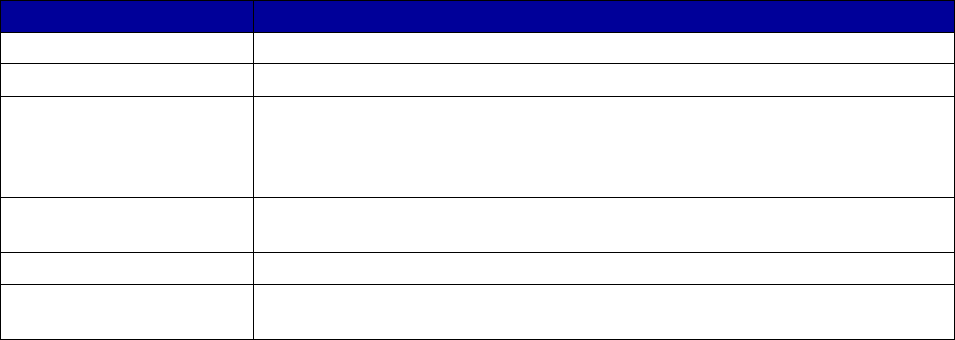
Coverage Versus Preferred Status
of MAT and Overdose Reversal Drugs
(number of states and territories)
Drug Coverage Preferred Status
• Acamprosate 40 27
• Buprenorphine 52 29
• Buprenorphine implant 37 2
• Buprenorphine injection
extended-release (ER) 33 7
• Buprenorphine-naloxone 52 51
• Disulfiram 49 32
• Methadone 42 —
• Naloxone 51 43
• Naltrexone (oral) 51 44
• Naltrexone ER 51 34
• Includes the 50 states, District of Columbia, Puerto Rico and the US
Virgin Islands
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
4
other MAT drugs. However, one state requires prior authorization for all drugs in the opioid
dependence treatment class.
As part of the authorization process, several states require evidence that the patient was
being referred or was concurrently receiving psychosocial treatment with their
medications. This requirement is most often applied to medications for opioid use disorders,
particularly buprenorphine and buprenorphine-naloxone.
Step therapy is another drug utilization management strategy that requires patients to try
a first-line medication, such as a generic medication, before they can receive a second-line
treatment, such as a brand name medication. This requirement is relatively common.
However, some states also impose step therapy requirements across medications types, in which
a drug may not be used unless one or more other specific drugs are tried unsuccessfully first.
One example might include requirements to try naltrexone before disulfiram or acamprosate.
Quantity or dosing limits often are used by Medicaid programs to avoid potential abuse or
misuse of a medication, promoting safe and appropriate medication use. Such limits are
used by 45 and 46 states for buprenorphine and buprenorphine-naloxone, respectively. In
addition to quantity limits, some states historically have established lifetime treatment limits,
most commonly applied to buprenorphine and buprenorphine-naloxone. However, lifetime limits
are disappearing, which is consistent with clinical evidence and best practices, given that
addiction is a chronic disease.
Key Findings on Innovative Approaches to Financing and Delivering MAT
States are using a variety of innovative approaches to finance and deliver medications for
alcohol and opioid use disorders. For example, Massachusetts is addressing the opioid
addiction epidemic by expanding medication access through a nurse care manager model. This
model allows physicians to treat more patients with buprenorphine and has proven to be cost-
effective. Another example is Missouri, which has begun the process of integrating MAT into
all SUD treatment in the state. Missouri currently is requiring any SUD treatment provider that
contracts with the state to offer MAT either directly or by referral. To ensure that providers are
fairly compensated for the provision of MAT, the state has taken steps to establish an equitable
reimbursement model that covers medications, the act of administration, laboratory services,
other MAT-related activities, and overhead costs. The state of Washington has implemented a
pilot involving a telemedicine project, Flex Care, at the Grays Harbor Clinic in the township of
Hoquiam (about 2 hours southeast of Seattle). Approximately 200 patients in rural coastal
Washington who previously had no access to MAT for their opioid dependence disorder now
receive MAT under the Flex Care treatment model.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
5
I. Introduction
Addiction is a chronic, relapsing brain disease that is characterized by compulsive drug or
alcohol seeking and use, despite harmful consequences (National Institute on Drug Abuse
[NIDA], 2016a). Drug-induced deaths have become a leading public health concern in the
United States, with opioid overdose and mortality rising at an especially alarming rate. It is
estimated that more than 72,000 Americans died from drug overdoses in 2017 and more than
63,000 in 2016 (NIDA, 2018). In 2016, more than 6 out of 10 of these deaths involved an opioid
(Seth et al., CDC MMWR, 2018). Opioid overdose
deaths (including both opioid pain relievers and
heroin) reached record levels in 2016, with an age-
adjusted 27.9 percent increase in just 1 year (Seth et
al., CDC MMWR, 2018). The use of illicit drugs,
including opioids, also is linked to a variety of
adverse health events (Devlin & Henry, 2008;
NIDA, 2012a).
Alcohol-related morbidity and mortality also present serious public health concerns. Each year
in the United States, an estimated 88,000 deaths are attributed to the use of alcohol (Centers for
Disease Control and Prevention, 2013). Excessive alcohol consumption is associated with
adverse health and social consequences, including liver cirrhosis, certain cancers, fetal alcohol
spectrum disorder, unintentional injuries, and violent behaviors (Bouchery, Harwood, Sacks,
Simon, & Brewer, 2011). Substance use disorder (SUD) also is associated with high costs to
both individuals and society (Substance Abuse and Mental Health Services Administration
[SAMHSA], 2014a).
Addiction, like many other chronic diseases, is a treatable condition. According to NIDA,
“treatment enables individuals to counteract addiction’s powerful effects on the brain and
behavior and allows them to regain control of their lives. According to research that tracks
individuals in treatment over extended periods, most people who get into and remain in treatment
stop using drugs, decrease their criminal activity, and improve their occupational, social, and
psychological functioning” (NIDA, 2012b). There are a variety of evidence-based approaches to
treating addiction. For alcohol and opioid use disorders, treatment can include psychosocial
treatments (such as cognitive-behavioral therapy or contingency management), medications, or a
combination of approaches. Although reliance on medication alone is not uncommon, a
combination of psychosocial treatment and medication generally is recommended for the
treatment of alcohol or opioid use disorders. Treatment that incorporates medication is referred
to as medication-assisted treatment or MAT. MAT for opioid use disorders using buprenorphine
or methadone is associated with substantial reductions in the risk for all cause and overdose
mortality (Sordo et al., 2017).
Substance Use-Related Deaths
There were over 63,000 drug
overdose deaths in 2016. Six out of
10 involved an opioid.
An estimated 88,000 deaths are
attributed to alcohol use annually.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
6
Medications can help individuals with SUDs re-establish normal brain functioning, prevent
relapse, and reduce cravings (NIDA, 2016b). The following medications have been approved by
the FDA for treatment of alcohol and opioid use disorders:
2
Alcohol use disorders—
• Acamprosate
• Disulfiram
• Naltrexone (oral)
• Naltrexone extended release
(injectable)
Opioid use disorders—
• Buprenorphine
• Buprenorphine extended release
(subdermal and injectable)
• Buprenorphine-naloxone
• Methadone
• Naltrexone (oral)
• Naltrexone extended release
(injectable)
In 2016, an estimated 21.0 million people aged 12 years or older in the United States needed
treatment for an illicit drug or alcohol use disorder (SAMHSA, 2017). SAMHSA found that only
10.6 percent of individuals (2.2 million people) who needed substance use treatment for illicit
drug or alcohol use had received it at a specialty facility in the past year. Among individuals
who recognized a need for treatment and tried to obtain it, lack of health coverage was the most
frequently reported reason (reported by 37.9 percent of those individuals) for not receiving
treatment (SAMHSA, 2017). Health insurance significantly increases access to SUD treatment
by making it more affordable. Medicaid is a critical payment source and is one of the largest
single payers of medications for treating SUDs. Medicaid was responsible for 25 percent of
SUD-related spending in 2014, and that share is projected to increase to 28 percent by 2020
(SAMHSA, 2014b). The primary purpose of this report is to present information about Medicaid
coverage of medications used to treat alcohol and opioid use disorders.
3
This report serves as an
update to the SAMHSA report Medicaid Coverage and Financing of Medications to Treat
Alcohol and Opioid Use Disorders (SAMHSA, 2014c).
In addition to describing the treatment and cost-effectiveness of these medications, the first
section of this report reviews policies and regulations that affect coverage of and access to them.
The second section describes Medicaid coverage of these medications for all 50 states, the
District of Columbia, Puerto Rico, and the U.S. Virgin Islands in the second and third quarters of
2018, and it explains the different benefit design elements most commonly used for each
medication. The third section provides examples of the way that states are using innovative
financing and delivery models to achieve positive outcomes and addresses some cross-cutting
best practices that may help increase access to SUD medications.
2
In addition, the Food and Drug Administration has approved the use of naloxone for the reversal of opioid
overdose.
3
Medications intended for the treatment of nicotine or tobacco use are excluded.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
7
II. State Considerations for Covering Medications
for Alcohol and Opioid Use Disorders
State Medicaid agencies typically use a Pharmacy and Therapeutics Committee or equivalent
body to (1) determine whether to provide reimbursement for new medications, (2) determine
whether to provide preferred or nonpreferred status for those medications, and (3) reconsider the
status of coverage of existing medications. Sometimes a separate drug utilization review
committee may contribute to the process. Usually, a new medication requires prior authorization
until a preferred or nonpreferred status is designated for the drug. Although priority reviews can
be enacted when appropriate, preferred or nonpreferred status and other coverage decisions can
generally take up to 6 months. The committee approval process often includes literature and
evidence review, efficacy determination, proposed protocol provisions, and safety and cost
considerations (American Society of Addiction Medicine, 2013).
This section presents topics that members of Pharmacy and Therapeutics Committees may consider
as they determine whether medications for alcohol or opioid use disorders will be available as
indicated on the Medicaid formulary or Preferred Drug List (PDL). These topics include evidence
regarding the treatment efficacy of these medications, their cost-effectiveness and cost offset, and
policies and regulations that may affect their coverage in Medicaid.
A. Efficacy of Medications Used to Treat Alcohol and Opioid Use Disorders
Before determining whether to provide coverage for a drug or whether to include it on a PDL, state
Medicaid agencies examine evidence of efficacy. These drugs are ones that the Food and Drug
Administration (FDA) already has approved for prescribing. It is important to note, however, that,
although the FDA approves drugs for certain indications, it does not decide how doctors use these
drugs or whether and to what extent Medicare,
Medicaid, and private insurers will cover drug costs.
Those are independent decisions made once the FDA
has approved the drug for release on the market.
The FDA evaluates a product’s safety and efficacy but
does not formally consider cost as part of the drug
approval process. The FDA approves drugs for
certain conditions or indications that then are reflected
on product labels. However, once these medications
are approved by the FDA, health care professionals
also may prescribe them “off-label” for other uses.
After receiving initial approval for treating one or
more given conditions, the manufacturer also may
subsequently seek FDA approval for use of the
Brand Versus Generic
Brand-name drugs are patented and
marketed by the manufacturer after
undergoing extensive research and
subsequent FDA approval. Once the
patent expires, other manufacturers
can produce generic equivalents, or
generics, that are required by the
FDA to be therapeutically equivalent
to the branded version. Generic
equivalents are typically priced much
lower than existing brand
medications.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
8
product for other indications or for a new route of administration or form (e.g., tablet or liquid) of
the medication (FDA, 2017a, 2017b). All the medications reviewed in this section are ones that
have met the FDA requirements for substantial evidence of safety and efficacy and were approved
by the FDA for the treatment of alcohol or opioid use disorders. After a brand-name drug’s
patents and other exclusivity have expired, a generic drug product may become available. The
FDA describes generic drugs as “copies of brand-name drugs and are the same as those brand
name drugs in dosage form, safety, strength, route of administration, quality, performance
characteristics and intended use” (FDA, 2017c). Although not always the case, generic drug
product prices may be up to 85 percent lower than their brand-name counterparts (FDA, 2017d).
For this reason, many PDLs will list generic drugs if one has been approved by FDA.
Medications for Alcohol Use Disorders
The medications currently approved by the FDA for treatment of alcohol use disorders are
acamprosate (also available as Campral®), disulfiram (also available as Antabuse®), oral naltrexone,
and extended-release injectable naltrexone (only available as Vivitrol®) (Table 1). Extended-release
injectable naltrexone and acamprosate were developed more recently than the other medications;
extended-release injectable naltrexone is not yet available in generic form. In general, scientific
research has found that these medications for alcohol use disorders help maintain abstinence, reduce
the risk of relapse, and reduce heavy drinking (Laaksonen, Koski-Jannes, Salaspuro, Ahtinen, &
Alho, 2008; Maisel, Blodgett, Wilbourne, Humphreys, & Finney, 2013; Mason & Lehert, 2012;
Specka, Heilmann, Lieb, & Scherbaum, 2014). Each is discussed briefly below.
Table 1. Medications Used to Treat Alcohol Use Disorders
Medication
Year of First
FDA
Approval
a
Mechanism of Action
Is a Generic
Version
Available?
Acamprosate
calcium (oral)
(Campral)
2004
Possible glutamate antagonist and gamma-
aminobutyric acid (GABA) agonist (not fully
known)—reduces symptoms of withdrawal
and craving
Yes
Disulfiram (oral)
(Antabuse)
1951
Alcohol antagonist—disulfiram plus alcohol
will produce flushing, throbbing in head and
neck, headache, nausea, vomiting, and other
highly unpleasant symptoms
Yes
Naltrexone (oral)
1994 (for
alcohol use
disorders)
Opioid antagonist—blocks opioid receptors
that are involved in alcohol and opioid
cravings
Yes
Naltrexone
(extended-release
injectable) (Vivitrol)
2006
Opioid antagonist—blocks opioid receptors
that are involved in alcohol and opioid
cravings
No
Abbreviations: FDA, Food and Drug Administration.
a
For more information on FDA approval of drugs, see U.S. Food and Drug Administration. Drugs@FDA:
FDA Approved Drug Products. Retrieved from http://www.accessdata.fda.gov/scripts/cder/daf/
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
9
Acamprosate. Acamprosate is an oral medication that is used for postwithdrawal maintenance
of alcohol abstinence. Short-term and long-term studies provide evidence of the efficacy of
acamprosate. For example, acamprosate is effective at increasing the cumulative days of
abstinence among individuals with alcohol use disorders (Bouza, Angeles, Ana, & María, 2004;
Donoghue et al., 2015; Maisel et al., 2013; Mann, Lehert, & Morgan, 2004; Rösner et al, 2011;
SAMHSA, 2009). This medication is associated with significantly higher rates of treatment
completion and medication compliance and has a significant effect compared with placebo in
improving rates of abstinence and no heavy drinking in people with alcohol use disorders
(Mason & Lehert, 2012). Acamprosate may be most effective among individuals who are
motivated for complete abstinence from alcohol and when provided over a long period of time.
Disulfiram. Disulfiram is the oldest medication used in the treatment of alcohol use disorder.
The medication, administered orally as a tablet, does not prevent alcohol craving; instead, it
deters subsequent alcohol consumption by causing unpleasant effects such as flushing, throbbing
headache, nausea, vomiting, and other unpleasant symptoms for 24–30 hours after taking the
medication. Research shows that, when taken consistently and under supervision, disulfiram
increases abstinence, prevents relapse, and decreases the frequency of alcohol consumption
(Brewer, Meyers, & Johnsen, 2000; SAMHSA, 2009; Specka, Heilmann, Lieb, & Scherbaum,
2014). The mechanism of action for disulfiram, an aversive reaction upon drinking alcohol,
may, however, lead to poor adherence. Consequently, expert consensus recommends using
disulfiram only with reliable and highly motivated individuals in monitored situations (in which
another person administers the medication) or in circumstances in which it is necessary to deter
an anticipated high-risk situation (Garbutt, 2009; Jorgensen, Pedersen, & Tonnesen, 2011; Mann,
2004; Suh, Pettinati, Kampman, & O’Brien, 2006).
Naltrexone (oral and injectable). Naltrexone is an opioid antagonist that is used to prevent the
reinforcing effects of alcohol and opioids. It has the advantages of not being addictive and not
reacting aversively with alcohol (Leavitt, 2002; National Institute on Alcohol Abuse and
Alcoholism, 2008). The FDA initially approved oral naltrexone for treating alcohol use
disorders in 1994.
In 2006, the FDA approved the injectable, extended-release formulation of naltrexone (known by
its trade name, Vivitrol) for the treatment of alcohol use disorder. This formulation is more
expensive than the oral form and is given once every 4 weeks rather than taken daily. Monthly
intramuscular injection has a clear advantage over the daily oral formulation because it is
clinically well-tolerated (occasional side effects in some patients may include pain and
tenderness at the injection site) and is more effective for patients who do not adhere well to a
daily oral regimen of naltrexone. Consistent bioavailability of the long-acting formulation

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
10
contributes to an improved adverse effect profile compared with its oral counterpart (Clapp,
2012; Mannelli, Peindl, Masand, & Patkar, 2007).
4
Many literature reviews and meta-analyses of randomized controlled trials have found that the
use of naltrexone for alcohol use disorders is effective at reducing the number of heavy drinking
days, alcohol-related mortality, and alcohol craving (Bouza et al., 2004; Garbutt et al., 2005;
Harris et al., 2015; Helstrom et al., 2016; Jonas et al., 2014; Lobmaier, Kunøe, Gossop, & Waal,
2011; Maisel et al., 2013; Pettinati et al., 2006; SAMHSA, 2009). Studies also have shown less
frequent relapses. It is commonly reported that naltrexone is most effective at significantly
improving drinking outcomes when the drug therapy is used in conjunction with psychosocial
support. In addition, its benefits are more pronounced for patients who stopped drinking alcohol
prior to entering treatment (lead-in abstinence). Genetic factors and adherence play a significant
role in the effectiveness of naltrexone (Chamorro et al., 2012; Volpicelli et al., 1997).
Medications for Opioid Use Disorders
Scientific research has established that treatment of opioid addiction with medication (1)
increases patient retention in treatment; (2) improves social functioning; and (3) decreases drug
use, infectious disease transmission, criminal activities, and the risk of overdose and death
(Connock et al., 2007; Gowing, Farrell, Bornemann, Sullivan, & Ali, 2011; Johnson et al., 2000;
Kinlock et al., 2007; Schwartz et al., 2013; Soyka, Zingg, Koller, & Kuefner, 2008; Thomas et
al., 2014; Woody et al., 2015; Zaric, Brandeau, & Barnett, 2000).
Medications currently approved for the management of opioid use disorders include
buprenorphine (available as Probuphine® [extended-release subdermal {implant}]), Sublocade®
[extended-release injectable], and in a sublingual formulation); buprenorphine-naloxone
(sublingual) (also available as Bunavail® [buccal], Suboxone® [sublingual], or Zubsolv®
[sublingual]); methadone (generally dispensed orally for MAT) (also available as Dolophine®)
oral naltrexone; and extended-release injectable naltrexone (only available as Vivitrol) (Table 2).
Generic versions of buprenorphine and buprenorphine-naloxone were made available in 2009
and 2013, respectively. Extended-release injectable naltrexone and the extended-release
subdermal and injectable forms of buprenorphine are the only medications for the treatment of
opioid use disorders that are not available in at least one generic form. Each of these
medications is discussed briefly below.
4
More information about extended-release injectable naltrexone is available on the FDA drug label
(https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021897
).
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
11
Table 2. Medications Used to Treat Opioid Use Disorders
Abbreviation: FDA, Food and Drug Administration.
Medication
Year of
First FDA
Approval
a
Mechanism of Action
Is a
Generic
Version
Available?
Buprenorphine
(sublingual)
2002
Partial-opioid agonist—attaches to opioid receptors
but produces only a limited opioid-like effect while
competitively inhibiting other opioids from attaching
to and fully activating the receptor. These
properties allow it to relieve withdrawal and reduce
cravings while blocking other opioids.
Yes
Buprenorphine
(subdermal/implant)
(Probuphine)
2016
Partial-opioid agonist—attaches to opioid receptors
but produces only a limited opioid-like effect while
competitively inhibiting other opioids from attaching
to and fully activating the receptor. These
properties allow it to relieve withdrawal and reduce
cravings while blocking other opioids for up to 6
months.
No
Buprenorphine
(extended-release
injectable)
(Sublocade)
2017
Partial-opioid agonist—attaches to opioid receptors
but produces only a limited opioid-like effect while
competitively inhibiting other opioids from attaching
to and fully activating the receptor. These
properties allow it to relieve withdrawal and reduce
cravings while blocking other opioids for up to a
month.
No
Buprenorphine/
naloxone (oral,
Bunavail [buccal],
Suboxone
[sublingual], Zubsolv
[sublingual])
2002
Opioid antagonist (naloxone) added to
buprenorphine to deter misuse by injection. If
buprenorphine/naloxone is injected, the user can
experience acute withdrawal.
Yes
Methadone (oral)
(Dolophine)
1947
Full-opioid agonist—attaches to opioid receptors
and produces a full range of opioid effects. Full
agonists differ from partial agonists in that, the
higher the dose, the greater the effect they produce.
Methadone is extremely long acting. When taken
daily at an effective dose, it relieves withdrawal and
reduces cravings. It also saturates the available
opioid receptors and inhibits the effects of other
opioids that may be ingested.
Yes
Naltrexone (oral)
1984 (for
opioid use
disorders)
Opioid antagonist— attaches to opioid receptors but
produces no opioid-like effect and prevents opioids
acting at the receptor.
Yes
Naltrexone
(extended-release
injectable) (Vivitrol)
2010
Opioid antagonist— attaches to opioid receptors but
produces no opioid-like effect and prevents opioids
acting at the receptor for up to a month.
No
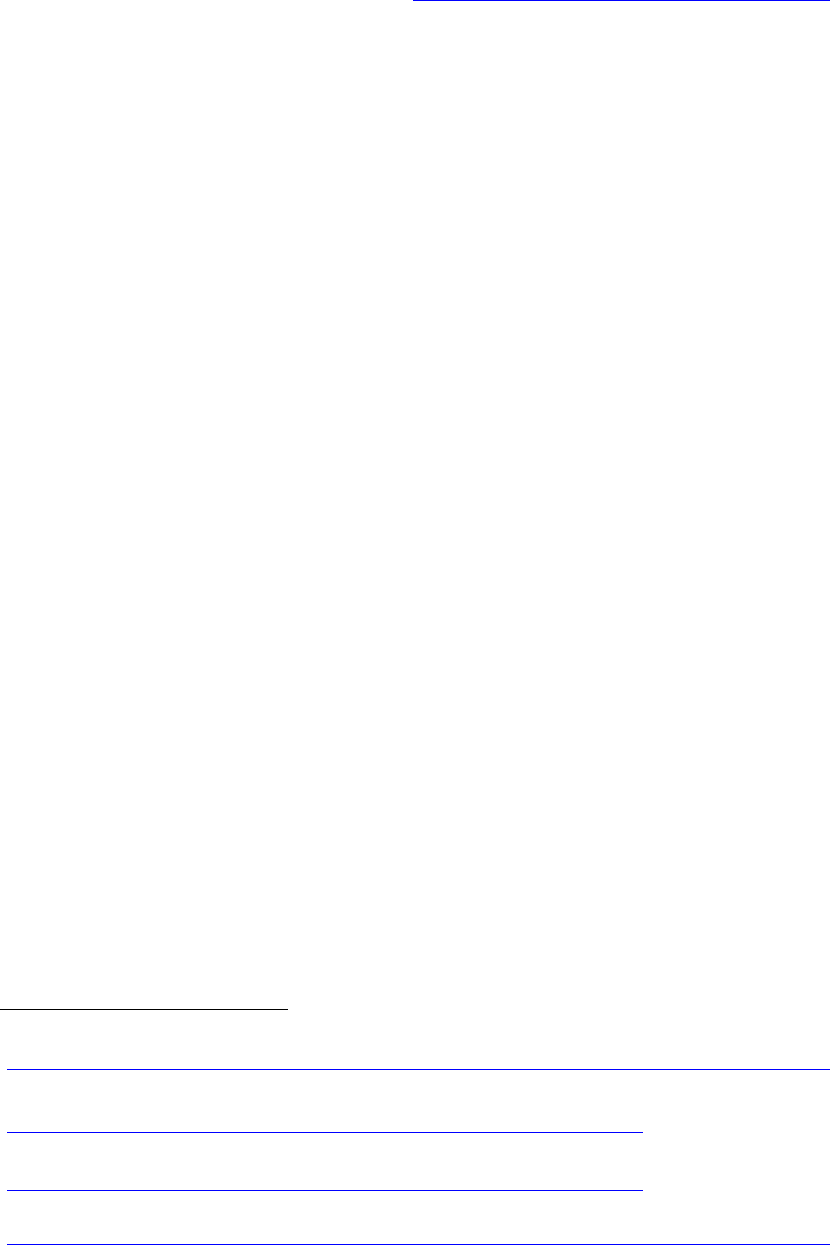
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
12
a
For more information on FDA approval of drugs, see U.S. Food & Drug Administration. Drugs@FDA:
FDA Approved Drug Products. Retrieved from http://www.accessdata.fda.gov/scripts/cder/daf/
Buprenorphine, buprenorphine extended-release, and buprenorphine-naloxone. Buprenorphine
5
is a partial opioid agonist. Buprenorphine alone, when administered sublingually, is indicated
for the induction phase of opioid use disorder treatment (National Library of Medicine, 2017) or
for maintenance therapy during pregnancy (SAMHSA, 2016a). A recently approved implantable
form of buprenorphine (Probuphine) is available for maintenance pharmacotherapy for opioid
use disorder for patients who have been stabilized at a low or moderate dose (FDA, 2016a).
6
An
extended-release injectable formulation (Sublocade) is available for treatment of moderate to
severe opioid use disorder in patients who have initiated treatment with a transmucosal
buprenorphine-containing product, and who have been on a stable dose for at least 7 days (FDA,
2017e).
7
Buprenorphine combined with naloxone
8
can be used for withdrawal and induction as well as for
the maintenance phase of treatment. FDA approved the oral generic form in 2013 (Formulary
Watch, 2013), but the medication also is marketed under the brand names of Bunavail (buccal),
Suboxone (sublingual), and Zubsolv (sublingual). Naloxone is an opioid antagonist widely used
as the antidote to opioid poisoning. It is combined with buprenorphine to reduce the risk of
buprenorphine being misused by injection. Naloxone is addressed in greater detail separately
regarding its use in managing opioid overdose.
Several comprehensive reviews have concluded that there is a high level of evidence from many
randomized clinical trials indicating that buprenorphine is a safe and effective treatment for
opioid use disorders (Amass et al, 2012; Mattick, Breen, Kimber, & Davoli, 2014; Thomas et al.,
2014). The American Society of Addiction Medicine (ASAM) National Practice Guidelines for
the Use of Medications in the Treatment of Addiction Involving Opioid Use (ASAM, 2015)
provides concrete guidance on the use of buprenorphine for induction and maintenance as well as
for use with special populations such as pregnant women.
Two recent studies provide information on the potential effects of insurer dose limits for
buprenorphine-naloxone use. The first study investigated the effect of dose limits paired with
prior authorization within the Massachusetts Medicaid program, in which the requirements for
prior authorization increased in frequency as dose limits increased (Clark et al., 2014). The
5
More information about buprenorphine is available on the FDA drug label
(https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=078633
).
6
More information about implantable buprenorphine is available on the FDA drug label
(https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204442s006lbl.pdf
).
7
More information about extended-release injectable buprenorphine is available on the FDA drug label
(https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209819s001lbl.pdf
).
8
More information about buprenorphine-naloxone is available on the FDA drug label
(https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=091149
).

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
13
results showed that the change effectively reduced the number of enrollees receiving high doses
but did not affect total health care cost for those patients. There was, however, a short-term
increase in relapses among individuals who were switched to lower doses, suggesting that the
change was initially difficult for certain patients. The second study conducted elsewhere found
that those with higher buprenorphine-naloxone doses had fewer aberrant drug tests and greater
retention in treatment than those required by a payer to reduce their dosage to 16 mg/day or
lower (Accurso & Rastegar, 2016).
Methadone. Maintenance treatment with methadone
9
has been used for many decades in the
United States. Pharmacologically, methadone is a full-opioid agonist in that it attaches to opioid
receptors and produces a full range of opioid effects, with greater effects at higher doses.
Methadone is extremely long acting (24–30 hours). When
taken daily at an effective dose, it relieves withdrawal and
reduces cravings. It also saturates the available opioid
receptors and inhibits the effects of other opioids that may
be ingested. Because the medication is taken orally and
has a slow and very long period of metabolism, it does not
generate the extreme euphoria of short-acting, injectable
opioids (e.g., heroin or many pharmaceutical opioids) in
properly prescribed doses (Rettig & Yarmolinsky, 1995).
Because methadone is an opioid and produces opioid
effects, however, its use for treatment is sometimes
controversial.
A high level of evidence from multiple randomized
controlled trials over the past 4 decades supports
methadone maintenance treatment (MMT) as an effective
method to reduce craving, use of opioids, and mortality.
Additionally, MMT usually improves health and social
functioning (Faggiano, Vigna-Taglianti, Versino, &
Lemma, 2003; Fullerton et al., 2014; Mattick, Breen,
Kimber, & Davoli, 2014; SAMHSA, 2012; Sordo et al.,
2017; Soyka et al., 2008).
Naltrexone (oral and injectable). The FDA first approved
naltrexone in 1984 as an oral agent for treating opioid use
disorders. Naltrexone is a long-acting opioid antagonist
that works by tightly binding to opioid receptors for 24–30
9
More information about methadone is available on the FDA drug label
(https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/017116s021lbl.pdf
).
Diversion Potential of
Buprenorphine
Policymakers have expressed
concern about the potential for
abuse of buprenorphine. In
making decisions about
coverage, it is important to weigh
the potential harm from diversion
against the consequences of
limiting access to effective
treatment. Poison control centers
and emergency departments
have reported that, among adults,
fewer emergency visits related to
buprenorphine reflect life-
threatening situations and result
in hospital admission than visits
related to the use of heroin,
methadone, or oxycodone
(Bronstein et al., 2009).
Buprenorphine-naloxone also has
been reformulated to an
individually packaged sublingual
film version in efforts to reduce its
potential for diversion as well as
its accidental use by children
(Clark & Baxter, 2013).
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
14
hours (oral) or up to 30 days (extended-release injection). This makes the opioid receptors
unavailable for activation should the individual subsequently take any opioid. Studies have
found that naltrexone
10
can be effective at decreasing relapse to illicit opioid use (SAMHSA,
2012); however, this result is dependent on adherence to treatment, which often is low for oral
naltrexone (Johansson, Berglund, & Lindgren, 2006; Swift, Oslin, Alexander, & Forman, 2011)
because those being treated must be abstinent to use the medication (O’Connor & Fiellin, 2000).
In 2010, FDA approved the extended-release injectable formulation of naltrexone
11
(Vivitrol) for
treating opioid use disorders. Studies have found that it produces significantly better retention in
treatment and lower rates of opioid relapse (Brooks et al., 2010; Lee et al., 2016) as well as
significantly lower opioid-related mortality compared with no treatment (Harris et al., 2015).
However, to prevent severe iatrogenic opioid withdrawal, patients must abstain from opioids for
a minimum of 7 days before beginning the naltrexone treatment; thus, it is effective when used
following medical detoxification from opioids or after a period of abstinence such as during
incarceration. Some literature suggests that counseling or other supports may be beneficial to
encourage continuation in treatment among some individuals receiving extended-release
injectable naltrexone (Brooks et al., 2010). Barriers to the use of extended-release injectable
naltrexone may include complexity of ordering and using the medication, cost, health plan
reimbursement policies, and lack of knowledge about the drug (Alanis-Hirsch et al., 2016).
Recent studies have shown that, although extended-release injectable naltrexone is effective for
preventing relapse to opioid use, its use is not as widespread compared with other
pharmacotherapies, in part because of cost and its more limited inclusion in payer formularies
(Lee, Kresina, Campopiano, Lubran, & Clark, 2015).
A recent randomized, multistate controlled clinical trial with criminal justice offenders
demonstrated effective results using extended-release injectable naltrexone to prevent opioid
relapse, with the use of this drug resulting in a longer median time to relapse compared with
usual treatment (Lee et al., 2016; NYU Langone Medical Center, 2016). It is important to
provide viable assistance to this population, which has a high potential for relapse, high risk of
mortality from drug overdose, and risk of repeated interactions with the criminal justice system.
These patients also are less likely to have access to other medications such as buprenorphine or
methadone, so extended-release injectable naltrexone may be the most effective treatment for
them.
Medications for Opioid Overdose
Naloxone. Naloxone is a short-acting opioid antagonist that has been demonstrated to be safe
and effective at reversing opioid-induced respiratory depression from opioid overdose (FDA,
10
More information about oral naltrexone is available on the FDA drug label
(https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018932s017lbl.pdf
).
11
More information about extended-release injectable naltrexone is available on the FDA drug label
(https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
).

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
15
2015; Hawk, Vaca, & D’Onofrio, 2015; van Dorp, Yassen, & Dahan, 2007). Naloxone currently
requires a prescription but has no abuse potential; it is not a controlled substance.
Over the past 40 years, naloxone has been used with good outcomes in the emergency
department and in hospital settings for opioid overdose reversal; it has an excellent safety profile,
and reported side effects have been rare (Burris, Norland, & Edlin, 2001; Davis, Ruiz, Glynn,
Picariello, & Walley, 2014). It initially was administered intravenously and later became
available intramuscularly (Evzio
®
). In 2015, the FDA approved the marketing of intranasally
administered naloxone (Narcan
®
). Increasingly, naloxone is being administered by
nonprofessionals in its intramuscular and intranasal forms (FDA, 2015), allowing administration
outside the hospital and emergency department by bystanders and professional first responders
(Mueller, Walley, Calcaterra, Glanz, & Binswanger, 2015; Wickramatilake et al., 2017). The
reader is referred to the SAMHSA Opioid Overdose Prevention Toolkit for more information
about naloxone (SAMHSA, 2016b).
Cost Offset and Cost Effectiveness of Medications for Alcohol and Opioid
Use Disorders
State Medicaid programs also examine the cost offset
and cost-effectiveness of medications used for alcohol
and opioid use disorders to determine whether to
reimburse for them and whether to place them on a
PDL. In doing so, states will take account of the high
costs associated with alcohol and opioid misuse and
use disorders.
Alcohol Use Disorder Costs
In 2010, excessive drinking costs the United States
were estimated at almost $250 billion annually
(Sacks, Gonzales, Bouchery, Tomedi, & Brewer,
2010). Studies of the comparative effectiveness of different treatments for alcohol use disorders
have found greater savings from use of MAT than from use of psychosocial treatment alone.
The following are findings from studies examining alcohol treatment, which relied on
retrospective claims data and did not distinguish those who received MAT alone from those who
received it in conjunction with counseling:
Total health care costs were 30 percent less for those who received MAT than for those
who did not (Baser, Chalk, Fiellin, & Gastfriend, 2011). This study compared costs for
those who received MAT with those who received only psychosocial therapy. Health
care costs were 34 percent greater for those receiving acamprosate than for those
receiving disulfiram, oral naltrexone, or extended-release injectable naltrexone.
Cost offset is defined as the
economic savings from an
intervention after accounting for the
economic costs of that intervention.
Cost-effectiveness is defined as
the comparison between the
relative costs and outcomes of an
intervention that is typically
quantified in an individual’s quality-
adjusted life years.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
16
Individuals treated with MAT compared with those who did not receive MAT incurred
less expense related to alcoholism-related inpatient hospitalizations and detoxification,
with extended-release injectable naltrexone reducing those costs the most (Mark,
Montejano, Kranzler, Chalk, & Gastfriend, 2010).
Use of extended-release injectable naltrexone resulted in the largest reduction in
nonpharmacy health care spending, compared with disulfiram, oral naltrexone,
acamprosate, or psychosocial therapy only (Bryson, McConnell, Korthuis, & McCarty,
2011).
Treatment with implanted naltrexone was associated with reduced health care events and
reduced costs of hospital admission and emergency department visits in patients treated
for alcohol use disorders in the first 6 months following treatment compared with those
who did not receive implanted naltrexone (Kelty et al., 2014).
A meta-analysis of studies indicated that health care utilization and costs were generally
equivalent to or lower for patients receiving extended-release injectable naltrexone
relative to patients receiving other alcohol use disorder agents (Hartung et al., 2014).
Opioid Use Disorder Costs
The economic consequences of opioid use disorders also are substantial. Annual health care
expenditures for individuals with an opioid use disorder are estimated to be almost nine times
higher than annual expenditures for those without such a disorder (White et al., 2005). The total
economic burden associated with fatal overdose, misuse, and use disorder attributable to
prescription opioid misuse in 2013 was estimated to be $78.5 billion, of which $28.9 billion was
associated with increased health care and SUD treatment costs and one-quarter was associated
with public sector health care, treatment, and criminal justice costs (Florence, Zhou, Luo, & Xu,
2016). A recent analysis in Australia also revealed substantial crime-related costs in that country
associated with heroin disorders (Dunlop et al., 2017).
As discussed below, cost-effectiveness and comparative effectiveness studies regarding opioid
treatment found greater savings from use of MAT than from use of psychosocial treatment alone.
Most of these studies, however, do not address whether those in the groups receiving MAT also
received counseling, although a study of the cost-effectiveness of adherence to buprenorphine
treatment did consider, among other things, the impact on outpatient treatment costs. These
studies relied primarily on claims data and one used predictive modeling but excluded the cost of
counseling. These studies are summarized below:
The odds and costs of hospitalizations, emergency department visits, and detoxification
admissions were greatest for those without treatment, followed by those treated without
medication, followed by those treated with buprenorphine, and finally followed by those
treated with methadone. In contrast, annual cost per patient was lower for those taking
buprenorphine than for those taking methadone largely because of longer hospitalizations
for those receiving methadone (Clark, Samnaliev, Baxter, & Leung, 2011).

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
17
Patients who did not receive pharmacotherapy had higher costs for detoxification,
rehabilitation, and both opioid-related and non-opioid-related hospitalizations. Among
those treated with medication, the highest drug costs were for extended-release injectable
naltrexone, whereas the highest overall costs were for methadone, apparently because of
a far higher number of non-opioid-related hospitalizations (Baser et al., 2011).
A meta-analysis of opioid dependent
extended-release injectable naltrexone
patients found they had lower inpatient
substance abuse-related utilization than
patients treated with other agents and
lower total cost than patients treated
with methadone (Hartung et al., 2014).
A study of the cost-effectiveness of
buprenorphine, which compared
individuals who were treatment
adherent with those who were not,
found that, although use of
buprenorphine resulted in increased
pharmacy costs ($6,156 vs. $3,581),
other costs—including outpatient
($9,288 vs. $14,570), inpatient
($10,982 vs. $26,470), emergency
department ($1,891 vs. $4,439), and
total health care costs ($28,458 vs.
$49,051)—were less (Tkacz,
Volpicelli, Un, & Ruetsch, 2014).
A study that modeled the incremental
cost-effectiveness of extended-release
injectable naltrexone, methadone, and
buprenorphine for adult males with
opioid dependence from the perspective
of state addiction treatment payers
found that the expected per patient cost
of a 24-week treatment period was
$1,390.98 for methadone, $1,837.40 for
buprenorphine, and $4,287.73 for
extended-release injectable naltrexone
(Jackson, Mandell, Johnson, Chatterjee,
& Vanness, 2015).
Controlled Substance Schedules
Substances deemed to be “controlled” under
the Controlled Substances Act are divided
into five schedules. Substances are placed in
their respective schedule on the basis of
whether they have a currently accepted
medical use in the United States, their relative
abuse potential, and their likelihood of
causing dependence when abused.
Controlled substances are overseen by the
Drug Enforcement Administration (DEA).
Drugs are scheduled by DEA in coordination
with the FDA.
Schedule I substances have no currently
accepted medical use in the United States, a
lack of accepted safety for use under medical
supervision, and a high potential for abuse.
Schedule II substances, which include
methadone and most opioid pain relievers,
have high potential for abuse and may lead to
severe psychological or physical dependence.
Schedule III substances, which include
buprenorphine, have less abuse potential
than those in Schedules I or II. Abuse may
lead to moderate or low physical dependence
or high psychological dependence.
Schedule IV substances have lower abuse
potential relative to those listed in Schedule III.
Schedule V substances have low abuse
potential relative to those listed in Schedule
IV, and they have preparations containing
limited quantities of certain narcotics.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
18
State and Federal Regulations and Policies Affecting the Prescription and
Dispensing of Medications for Alcohol and Opioid Use Disorders
In addition to considerations related to efficacy and cost-effectiveness, federal and state laws and
other policies may affect the prescribing and dispensing of medications for alcohol and opioid
use disorders. In this section, we address some of the most important statutes, regulations, and
other policies.
Federal Laws Governing Methadone and Buprenorphine
Medications for treatment of alcohol and opioid use disorders must be prescribed or dispensed by
individuals who are licensed to perform these activities in their respective states; however,
additional rules and regulations apply to methadone and buprenorphine because of their status as
controlled substances under the Comprehensive Drug Abuse Prevention and Control Act
(Controlled Substances Act, 1970). The rules and regulations affect access to these medications
regardless of insurance coverage.
Methadone is a Schedule II drug that is used for the treatment of both pain and opioid addiction
(U.S. Department of Justice, n.d.). When prescribed for pain, it may be dispensed by a
pharmacy. For treating opioid addiction, however, methadone may be dispensed only through an
opioid treatment program (OTP) that has been certified by SAMHSA and registered as a narcotic
treatment program by the U.S. Drug Enforcement Agency (DEA) (SAMHSA, 2015).
Buprenorphine is a Schedule III drug (U.S. Department of Justice, n.d.), indicating its lower
potential for abuse or misuse than Schedule II substances. Pursuant to the Drug Addiction
Treatment Act of 2000 (DATA 2000), qualified physicians can prescribe buprenorphine to
patients for the treatment of opioid use disorder after completing a required training and
submitting to SAMHSA a notification of intent to prescribe. This permitted the physician to
treat up to 30 patients at a time in the first year and, if requested, 100 patients at a time after that
(SAMHSA, 2016c). In July 2016, a regulation was finalized that created the possibility for some
physicians with added qualification or in specific practice settings to treat up to 275 patients at a
time. Later in 2016, the Comprehensive Addiction and Recovery Act of 2016 (CARA) amended
the Controlled Substances Act to allow qualifying nurse practitioners and physician assistants to
receive a DATA 2000 waiver and prescribe buprenorphine at the original 30 and 100 patient
limits. As discussed in the section on state laws and policies, several states have scope of
practice laws that limit the effect of this federal law. In October 2018, President Trump signed
into law the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment
(SUPPORT) Act. This law contains provisions intended to increase access to and use of MAT.
Table 3 summarizes federal prescribing restrictions that apply to alcohol and opioid use disorder
treatment. Relevant state laws or regulations are discussed in the section on state laws and
policies.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
19
Table 3. Federal Prescribing Regulations for Medications Used to Treat Alcohol and
Opioid Use Disorders or to Reverse Opioid Overdose
Medication Federal Restrictions
Acamprosate Can be prescribed by a licensed health care professional or practitioner
Disulfiram Can be prescribed by a licensed health care professional or practitioner
Buprenorphine/
buprenorphine-naloxone
Can be prescribed by a physician, nurse practitioner, or physician assistant
to up to 30 or 100 patients at a time after completing required training.
Some physicians with added qualification or in specific practices may treat
up to 275 patients at a time.
Methadone
Can be administered only by a SAMHSA-certified opioid treatment program
that has been registered with DEA as a narcotic treatment program
Naloxone Can be prescribed by a licensed health care professional or practitioner
Naltrexone (oral and
injectable)
Can be prescribed by a licensed health care professional or practitioner
Abbreviations: DEA, Drug Enforcement Administration; SAMHSA, Substance Abuse and Mental Health
Services Administration.
Requirements of Parity
Additional laws and policies affect access to medications for alcohol and opioid use disorders.
The Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act
(MHPAEA) of 2008 requires that the cost sharing and treatment limitations for medications used
to treat SUDs, if covered by a health plan, must be comparable to and no more restrictive than
medications for other medical or surgical needs (Center for Consumer Information and Insurance
Oversight, 2013). These requirements apply to both quantitative and nonquantitative treatment
limits (NQTLs), which include some of the utilization management techniques commonly
applied to MAT medications (e.g., prior authorization and step therapy). Federal parity law
prohibits the use of any NQTLs for mental health or SUD benefits unless the processes,
strategies, evidentiary standards, or other factors used in applying the NQTLs to the behavioral
health benefits in the classification are comparable to, and applied no more stringently than, the
processes, strategies, evidentiary standards, or other factors used in applying the NQTLs to
medical benefits in the same benefit classification (e.g., the prescription drug benefit
classification). Thus, for example, this MHPAEA requirement is satisfied if health plans use a
tiered formulary, in which different financial requirements and treatment limits are imposed
uniformly for different tiers of drugs on the basis of factors unrelated to diagnosis, such as the
cost and efficacy of the drug (Department of the Treasury, 2013). In March 2016, the Centers
for Medicare & Medicaid Services (CMS) released a Final Rule implementing the MHPAEA
requirements for the Children’s Health Insurance Program, the Medicaid benchmark benefit
plans, and Medicaid managed care plans (Department of Health and Human Services, 2016).
Separate but parallel regulations implement MHPAEA for nonfederal government plans with
more than 100 employees and group health plans of private employers with more than 50
employees (Center for Consumer Information and Insurance Oversight, 2013).

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
20
States also have implemented parity laws that vary greatly regarding required coverage. As
movement occurs toward enforcement of the federal parity laws, states also are enacting statutes
and promulgating regulations designed to meet the federal requirements (National Conference of
State Legislatures, 2015; ParityTrack, n.d.).
State Laws and Policies
In addition to the state parity laws, state laws and policies may affect access to or reimbursement
of high-quality MAT in other ways. State laws fall into several categories. In a recent report on
the integration of physical and behavioral health services, with particular focus on Medicaid,
Bachrach and colleagues (2014) identified four types of statutes or regulations that may impede
the provision of integrated care, including behavioral health. These requirements included ones
related to (1) professional licensure and certification, (2) facility licensing and certification, (3)
billing requirements, and (4) data exchange (Bachrach, Anthony, & Detty, 2014). Each of these
categories of regulation may affect the provision of integrated care that includes MAT, and some
may influence the ability to provide MAT per se. Each of these requirements as well as other
state policies that may affect the provision of MAT or naloxone are addressed briefly below.
Professional Licensure and Certification
Given the shortage of physicians and, particularly, physicians approved to prescribe
buprenorphine in some areas, it is expected that treatment facilities will take advantage of the
2016 statutory and regulatory changes and turn to physician assistants or nurse practitioners to
fill the void. Individual state laws, however, may restrict whether and when a physician assistant
or nurse practitioner may prescribe medication within the scope of his or her license and the level
and type of nursing license required. State regulations may limit further whether a nurse
practitioner may prescribe controlled substances and may limit such drugs to certain schedules,
may limit the age of patients for which prescribing is allowed, may place time limits on the
prescription (e.g., 7 days), may limit prescribing setting (e.g., only inpatient), or may impose
other prescribing restrictions specifically applicable to nonphysician prescribers (American
Association of Nurse Practitioners, 2017; Stowkowski, 2016). Such limits have particularly
profound effects on the prescribing of buprenorphine, which has strict federal prescribing
restrictions that limit the availability of approved prescribers.
As discussed above, in its July 8, 2016, rulemaking, SAMHSA expanded the number of patients
to whom a waivered provider may prescribe buprenorphine (Medication Assisted Treatment for
Opioid Use Disorders, 2016). Subsequently, Congress amended the enabling statute to permit
both physician assistants and nurse practitioners to become waivered to prescribe buprenorphine
upon completion of additional instruction as part of certification (Comprehensive Addiction and
Recovery Act of 2016, 2016). As of April 2017, however, 28 states had scope of practice laws
that prohibit nurse practitioners from prescribing buprenorphine without a waivered physician’s
supervision. These restrictions may preclude certain states from taking full advantage of the
2016 changes to the federal laws governing buprenorphine prescribing by nonphysicians.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
21
Additionally, as of April 2017, three states explicitly prohibited nurse practitioners from
prescribing buprenorphine, even when working with a waivered physician, and an additional
state prohibited physician assistants from prescribing buprenorphine (Vestal, 2017). These state
requirements mean that the extent of nonphysician prescribing of buprenorphine will vary by
state.
Facility Licensing and Certification
Facility licensing or certification regulations in some states historically have required facilities
providing SUD treatment and any other physical or mental health treatment to be separate—for
example, requiring separate entrances, waiting rooms, or bathrooms. In addition, county or
municipal laws may restrict siting of opioid treatment facilities. Some states recently have taken
steps to eliminate some of these requirements (e.g., Arizona [Bachrach et al., 2014]), to clarify
that such requirements do not apply (e.g., Texas [Texas Health and Human Services, 2016]), or
to allow for waivers of such requirements (e.g., Massachusetts [Bachrach et al., 2014; Behavioral
Health Integration Task Force, 2013]). To the extent that such requirements exist, however, they
may impede comprehensive care, care coordination, warm hand-offs, and other aspects of
integration that can be integral to the provision of SUD treatment, including the provision of
MAT in conjunction with other services addressing physical or mental health. Such restrictions
also may reinforce discrimination in that they separate SUD treatment, identifying both the
disorder and its treatment as distinct from other conditions, including other chronic illnesses.
Billing Requirements
Billing procedures and requirements can affect access to MAT. For example, in the context of
integrated care, some states restrict whether a provider may bill for both a physical and a
behavioral health visit in 1 day, limiting the ability to provide convenient integrated care for
those with comorbid conditions (Bachrach et al., 2014; Brolin et al., 2012). Such billing
restrictions also may affect the provision of MAT integrated with other services. For example, a
primary care physician providing buprenorphine for an opioid use disorder diagnosis may be
prohibited from providing accompanying physical health care, requiring separate appointments
on separate days. Requiring separate appointments may discourage the patient from obtaining
complete care and may increase the cost of care to both the patient and the Medicaid program.
Some states also limit the types of providers who may bill for behavioral health services or the
types of procedures for which they may bill. They also may limit diagnosis codes for which
primary care providers may receive reimbursement under Medicaid (Bachrach et al., 2014;
Mauch, Kautz, & Smith, 2008). This may lead providers to record a physical health diagnosis
rather than a nonreimbursable behavioral health diagnosis, resulting in inaccurate records and
confusion for providers seeking to provide coordinated care (Bachrach et al., 2014). Partial
information in patient records may substantially impede coordination of care with other
providers and may produce questionable data for quality improvement, performance
measurement, and research.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
22
Data Exchange
Bachrach et al. (2014) identified a regulatory impediment to data exchange that may adversely
affect integrated care, potentially including the provision of MAT. This impediment involves
requirements for privacy and confidentiality regarding SUDs, particularly as exemplified by
federal regulations at 42 CFR Part 2 (Bachrach et al., 2014). Although 42 CFR Part 2 was
amended in 2016 to relax somewhat the prior restrictions, it is too early to determine what
practical effect this amendment will have. Under the earlier version of the regulation, however,
states interpreted the restrictions differently, with greater and lesser degrees of caution; some
states also have separate privacy restrictions that may differ from and be stricter than the federal
regulation. It remains to be seen how state laws and varied state interpretations of the amended
federal regulation will affect data exchange and related care integration and coordination,
including those involving MAT.
Other Policies That May Affect Delivery of MAT
Adequacy and timeliness of provider reimbursement. Adequacy and timeliness of provider
reimbursement are factors that affect whether providers accept Medicaid patients (Cunningham
& Malley, 2009). State Medicaid reimbursement rates for MAT vary greatly, and although many
states are working to increase delivery of MAT, others have cut reimbursement rates, which
some believe has made access more difficult (Terkel & Cherkis, 2015). Anecdotal evidence
suggests that Medicaid reimbursement for some MAT medications is below cost in certain states,
which may discourage the provision of services to Medicaid beneficiaries, undermining access to
MAT and other treatment.
Laws and policies affecting naloxone availability. Of the medications discussed in this report,
naloxone is unique. It is not MAT per se but is used to reverse opioid overdoses. The branded
versions of naloxone can be expensive, and price increases have made access difficult (Gupta,
Shaw, & Ross, 2016), including for agencies that must plan, budget, and pay for supplies of the
medication. States are implementing creative approaches both to make naloxone readily
available to those who need it and to provide ways to pay for it. The following are some
approaches that states and municipalities around the country are taking to disseminate naloxone
as broadly as possible:
Making naloxone or a naloxone prescription available in OTPs for individuals receiving
methadone or buprenorphine from the OTP. States also are increasingly reaching out at
other locations where those at risk of overdose are likely to be found, such as needle
exchange programs (Addiction Treatment Forum, 2014; Drug Policy Alignment, n.d.;
Oregon Health and Science University, 2015).
Distributing naloxone through Opioid Overdose Prevention Programs, emergency
responders, SUD treatment providers, community pharmacists, local health department
offices, recovery homes, sober houses, assertive community treatment teams, mobile
crisis teams, case managers, recovery support specialists, school personnel, and staff
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
23
within the criminal justice system (e.g., Drug Policy Alignment, n.d.; New York State
Department of Health, n.d.; U.S. News and World Report, 2016).
Federal agencies also have taken steps to facilitate naloxone access. Two examples include the
SAMHSA Opioid Overdose Prevention Toolkit and the FDA Naloxone App Competition (FDA,
2016b; SAMHSA, 2016b). The SAMHSA Opioid Overdose Prevention Toolkit provides
valuable information on some of the key approaches being undertaken to date, including
coprescribing naloxone to people who receive opioids for pain or opioid use disorder treatment,
dispensing naloxone to persons completing detox and rehab or being released from jail,
furnishing professional first responders such as law enforcement officers and emergency medical
technicians with naloxone, and implementing standing orders for pharmacies to dispense
naloxone to people at risk of an overdose (SAMHSA, 2016b). The FDA Naloxone App
Competition was created to promote creative technological approaches to making naloxone
accessible, with a prize awarded to the team designing the selected app (FDA, 2016b).
States also have passed laws, promulgated regulations, and taken other administrative or
executive actions allowing or encouraging necessary naloxone use, such as by (1) enacting
legislation establishing statewide “standing orders” for naloxone dispensing or providing
authority for more limited standing orders (e.g., California Legislative Information, n.d.; Justia,
n.d.); (2) taking executive action to issue statewide standing orders (e.g., Commonwealth of
Pennsylvania, 2015); (3) granting prescribing privileges to pharmacists (e.g., North Dakota
Department of Human Services, 2016; Oregon State Legislature, 2016); (4) depending on the
state, allowing different categories of professionals to administer naloxone pursuant to a standing
order (e.g., law enforcement personnel, fire department personnel, and emergency medical
personnel [State of Delaware, 2014; State of South Dakota, 2015]); and (5) allowing third parties
such as families or caregivers to be dispensed naloxone and granting “Good Samaritan” status to
those who administer it in the event of an overdose (e.g., CMS, 2017a; Harm Reduction
Coalition, n.d.; Legal Systems, LLC, 2016; Muller, 2016; National Conference of State
Legislatures, 2017; Policy Surveillance Program, 2016).
State Medicaid programs have taken steps to reimburse for naloxone, as do some but not all
other payers (Appendix A, Table A-10). In addition to insurance reimbursement, states use
alternate funding sources and approaches to pay for naloxone, such as the following:
SAMHSA-funded Prescription Drug Overdose discretionary grants
SAMHSA-funded State Targeted Response Opioid Crisis Grants
SAMHSA-funded Substance Abuse Block Grants
State funding of local health departments
State mental health and SUD agency funding
State general funds
Funding through municipality or other community budgets
Pharmaceutical company funding and pharmaceutical company settlement funds
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
24
Naloxone manufacturer rebates to health departments, emergency personnel, and other
entities
The use of bulk purchases to lower cost
When Medicaid does pay for naloxone, the process typically works like any other covered
medication. An enrollee is directly dispensed prescribed medication covered by the plan, or
another individual picks up the medication on the enrollee’s behalf, with the prescription having
been filled for the enrollee and paid for by his or her Medicaid account. The promulgation of
laws permitting dispensing of naloxone without a traditional prescription (under standing orders)
or permitting dispensing to someone other than the end user (e.g., family or friends) introduces
the question of how the naloxone is reimbursed if the traditional link between prescriber,
dispenser, and end user is altered in some way. Nearly all state Medicaid programs still envision
that the end user of naloxone will be the Medicaid beneficiary under whose Medicaid account
payment is made. This approach, in concert with laws that facilitate the use of naloxone by
Good Samaritans, may have an unintended consequence whereby Medicaid beneficiaries use the
naloxone that they purchased with Medicaid benefits to revive others who may or may not be
Medicaid beneficiaries.
As of October 2016, however, two state Medicaid programs appear to have modified the
standard reimbursement process and were reimbursing third parties for naloxone purchases
without any active involvement by the beneficiary (Smith et al., 2016). Our team identified one
of these states is New York, which reimburses as part of its fee-for-service (FFS) Medicaid
program. New York law defines an “opioid antagonist recipient” as “a person at risk of
experiencing an opioid-related overdose, or a family member, friend or other person in a position
to assist a person experiencing or at risk of experiencing an opioid-related overdose, or an
organization registered as an opioid overdose prevention program pursuant to this section or a
[series of school entities]” and allows the dispensing of naloxone to such persons or entities
(New York State Public Health Law, Article 33 [Justia, 2016]). Pharmacists operating under a
standing order may bill FFS Medicaid for that medication, if the intended user is not dually-
eligible for Medicare and Medicaid and is a Medicaid fee-for-service enrollee (New York
Department of Health, 2016). This suggests that any of the individuals or entities enumerated in
the definition of an “opioid antagonist recipient” actually may purchase the naloxone and have it
billed to Medicaid. In contrast to the approach that New York has taken as part of its FFS
Medicaid program, what is more likely to happen in other states is that the opioid user who is a
Medicaid beneficiary will be prescribed and dispensed the medication to take home, where it
later may be administered by others if the need arises.
On January 17, 2017, CMS issued an informational bulletin that highlighted some of the options
that states have for the improved use of pharmacists to expand timely access to certain drugs in
the interest of public health, specifically including naloxone. These options included expanding
the scope of practices and range of services that pharmacists can provide, “including dispensing
drugs based on their own independently initiated prescriptions, collaborative practice agreements
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
25
(CPA) with other licensed prescribing healthcare providers like physicians, ‘standing orders’
issued by the state [health authority], or other predetermined protocols” (CMS, 2017b, p. 1).
Most states use one or more of these approaches to increase access to a variety of medications
such as naloxone, nicotine replacement therapy, emergency contraception, or flu shots. The
CMS bulletin discusses the value of using these approaches for time-sensitive medication dosing,
such as naloxone, and encourages states to consider how they might use these methods for
addressing public health emergencies (CMS, 2017b). CMS did not, however, address the issue
of Medicaid coverage as it might apply to dispensing to third parties. It seems that this is to be
left to each state to consider and possibly implement if it is considered useful by the state in
stemming the tide of opioid overdose fatalities.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
26
III. Medicaid Coverage of Medications for Alcohol and
Opioid Use Disorders
This section summarizes state Medicaid coverage policies for medications used to treat alcohol
and opioid use disorders in the 50 states, the District of Columbia, Puerto Rico, and the U.S.
Virgin Islands. Information is presented about whether a medication is reimbursed and whether
it has an identifiable preferred status. Requirements for prior authorization, quantity limits,
treatment duration limits, step therapy, and concurrent behavioral therapy also are described.
Detailed results of the analyses are outlined by drug and state in Appendix A.
A. Data Sources and Methodology
Medications specifically researched include the following:
Acamprosate
Buprenorphine
Buprenorphine, implantable
Buprenorphine, extended-release injectable
Buprenorphine/naloxone
Disulfiram
Methadone
Naloxone
Naltrexone, oral
Naltrexone, extended-release injectable
Rules for Determining Coverage
The research team used multiple data sources to obtain information on Medicaid coverage of
MAT.
12
During the second and third quarters of 2018, the most recent pharmacy and behavioral
health documents were retrieved from state Medicaid agency websites. Documents were
reviewed to identify information related to Medicaid pharmaceutical coverage of and benefit
design for MAT, including, where available, selected Medicaid managed care plans. Internet
queries sought documents providing information on formularies, PDLs, prior authorization,
behavioral therapy requirements, quantity limits, and other pharmacy benefit limitations. Where
ambiguity remained, online drug search tools such as Epocrates®
(https://online.epocrates.com/rxmain) were accessed, which provided additional information that
allowed us to pursue the subject further using other documents. Medications may be covered by
state Medicaid programs without being listed on an easily accessible formulary; therefore, when
there was no clear evidence of coverage in the Medicaid or other documents, a determination of
12
Our methods for determining state coverage of methadone for SUD treatment are discussed separately below.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
27
coverage was made by searching the 2018 Medicaid State Drug Utilization Data (CMS, n.d.).
These data identify medications for alcohol and opioid use disorders that are paid by Medicaid
during one quarter for each year. If the Medicaid programs paid for an alcohol or opioid use
disorder medication during the reported quarter of 2018, then the state was classified as covering
the drug. Puerto Rico and the U.S. Virgin Islands are not included in this dataset, so coverage
for those two territories could not be verified. Additionally, for extended-release naltrexone
(Vivitrol), implantable buprenorphine (Probuphine), and extended-release buprenorphine
(Sublocade), which are not consumed orally, if a determination of coverage could not be made
from any of the above sources, we searched state provider fee codes to determine whether the
drug was available only as a medical benefit. The codes used were as follows:
Buprenorphine, implantable: J0570
Buprenorphine, extended-release injectable: Q9991, Q9992
Naltrexone, extended-release injectable: J2315
In all instances, whether regarding reimbursement, preferred status, or benefit limitations, if a
definitive determination of a state’s coverage policy could not be made for a particular
medication, the coverage was treated as unknown. The list of documents used for this review can
be found in Appendix A, Table A-1. Where necessary for clarity, additional details on benefit
designs within specific states are outlined in the notes to the appendix tables.
Rules for Determining Preferred Status and Benefit Limitations
Inclusion in the Medicaid State Drug Utilization Data does not necessarily indicate that the
drug is covered both by a state’s FFS Medicaid plan and by Medicaid managed care
organizations (MCOs). It also does not indicate whether the drug has preferred status or is on
a state’s PDL. Similarly, exclusion from a PDL does not necessarily mean that the drug is not
covered or even not preferred. It is important to note that not all states have a PDL. Moreover,
state PDLs have different designs. For that reason, drugs were identified as having a
“preferred status” rather than as being on the state’s PDL. Following are the issues observed
in the data regarding PDL inclusion, along with how assigning preferred status to medications
was addressed:
A PDL may list a drug and indicate that it is either preferred or nonpreferred. The
requirements for nonpreferred drugs typically include prior authorization and sometimes
other requirements. Even preferred drugs may have these requirements. If a drug was
identified as preferred on a PDL, it was treated as preferred. If a generic version was
identified as preferred and the brand version as nonpreferred, the medication was treated
as preferred.
A PDL may list only certain categories or classes of drugs. In these instances, a specific
drug may be—
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
28
o In a drug category that is not included on the PDL. If so, the PDL may state that
such a drug requires prior authorization, may state that a drug does not require
prior authorization, or may be ambiguous. If the PDL stated that those omitted
categories do not require prior authorization, the drug was treated as preferred.
o In a drug category that is included and listed within that drug category. If so,
inclusion on the PDL may mean that the drug is automatically preferred, or the
PDL may state that certain drugs in the included categories are preferred or not,
and typically include whether prior authorization is required. If a drug was
identified as preferred, it was treated as such.
o In a drug category that is included but not listed within that category. If so, the
PDL may indicate that prior authorization is required, or it may be ambiguous. In
that instance, the drug was treated as nonpreferred.
There may be a separate document related to specific categories of drugs (e.g., MAT
medications) indicating exactly what benefits and limitations apply. If the drug was
included without limitation, it was treated as preferred.
There may be a separate computerized “look-up” that serves as the most current
formulary, or even the current PDL, and that provides information on coverage and
benefit limits. If the drug was included without limitation, it was treated as preferred
unless there was any indication to the contrary on a PDL.
A state’s Medicaid formulary or PDL may apply only to FFS coverage or may apply to
both FFS and MCO coverage. Some MCOs may have independent formularies or PDLs
with their own requirements for preferred status, prior authorization, or other benefit
limitations. There may be one or many MCOs in a state, each with different
requirements. If the research team could locate at least one MCO where the drug fit the
criteria set out above as preferred, the drug was identified as such for that state. No
attempt was made to try to identify every Medicaid MCO in a state or to locate applicable
formularies or PDLs for each MCO. Rather, one or more MCOs were arbitrarily
selected, and the same process applied to those MCOs as was done for any information
available for FFS coverage.
Finally, for buprenorphine-naloxone, we compared the results we obtained from our
review of Medicaid documents current as of 2018 against the CMS Medicaid Drug
Utilization Review State Comparison/Summary Report FFY 2017 Annual Report on
Prescription Drug Fee-For-Service Programs. That report includes information on
whether any formulation of buprenorphine-naloxone was treated as preferred by each
state’s FFS Medicaid program during the fiscal year ending September 30, 2017. This
resulted in us adding one state as giving preferred status to buprenorphine-naloxone
where we had been unable to determine this from review of Medicaid documents in the
state. All other inconsistencies were attributable to the fact that our review of state
documents was more current than the information in the DUR report and we retained our
original decisions in those instances. The DUR report was also used to determine

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
29
requirements for quantity limits for buprenorphine and buprenorphine-naloxone
(excluding buprenorphine implantable or long-acting injectable formulations) in instances
where our review of the state Medicaid documents publicly-available in 2018 did not
provide certainty about those requirements (CMS, 2018).
The differences across states and between Medicaid payers within a state make it (1) challenging
to determine the meaning of inclusion on (or exclusion from) any one PDL and (2) difficult to
judge with high precision whether prior authorization is required or other limits are imposed. In
determining prior authorization status, if at least one Medicaid insurer required prior
authorization (other than for brand in contrast to generic drugs), the drug was treated as requiring
prior authorization. Similarly, if at least one Medicaid insurer required counseling, step therapy,
or quantity limits, it is indicated that the limit was required, even though it may not be true of all
Medicaid payers in the state and does not indicate that this is the policy of that state’s Medicaid
program. If the status of prior authorization was unclear because the drug was not included on a
limited PDL, the prior authorization status was treated as unknown. To make the decisions as
clear as possible, notes were placed at the end of each appendix table where further clarification
was needed. The status of benefits and limitations was delineated as clearly and accurately as
possible, although there was uncertainty about the status in certain cases. Additionally, the status
may change over time within a given state. Readers are encouraged to consult recent
documents applicable to the state and Medicaid payer of interest for the most current and
clear statement of coverage and limitations.
Some states limit the number of prescriptions for which a Medicaid enrollee may be reimbursed
in a month. Those limits were not included as quantity limits because they apply to all
medications, and they are not specific to the drugs being studied in this report. However, such
limits also may affect access to MAT.
Approach to Analysis of Methadone Coverage
Because methadone for SUD treatment is not prescribed and dispensed in the same manner as all
other MAT medications, it is difficult to ascertain whether it is covered by Medicaid for
treatment of opioid use disorder in each state. This is further complicated by the fact that
methadone also is prescribed for pain and that, when covered by a state Medicaid program, its
inclusion on formulary, preferred drug, or prior authorization lists relates to its use for pain.
13
For that reason, the research team used a different approach to ascertain methadone coverage
than was used for all other types of MAT. The following approach was used to determine
whether the dispensing of methadone as MAT was included in a state’s Medicaid reimbursement
for SUD treatment and, if so, what limits might have applied.
13
Please see discussion of results related to methadone for an explanation of the very limited circumstances in
which prior authorization or quantity limits may apply to the administration of methadone as MAT.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
30
Determination of coverage. To determine whether a state program covered methadone, the
researchers followed the process listed below:
Step one. First, state Medicaid documents were examined to determine whether any of
the following billing codes were identified as reimbursed:
o H0020: Alcohol and/or drug services; methadone administration and/or service
(provision of the drug by a licensed program)
o S0109: Methadone, oral, 5 mg
If at least one of these codes was included in at least one state Medicaid FFS plan
document, methadone was treated as reimbursed for SUD treatment.
Step two. If not clear from step one, other state Medicaid documents were examined to
determine whether methadone was reimbursed for SUD treatment. If other Medicaid
documents were unambiguous about coverage, it was treated as clearly covered or not
covered, depending on the document content. In some cases, the team relied on state
Medicaid statutes or regulations and state Medicaid Section 1115 documents—the latter
for states participating in Section 1115 demonstrations related to the provision of SUD
treatment.
Step three. The 2013 report sponsored by ASAM, on which researchers relied to
ascertain coverage of methadone for the 2014 version of this report, also was revisited as
a final check on coverage (ASAM, 2013). The 2013 ASAM report provided the results
of ASAM’s survey of Medicaid directors, which asked specifically about coverage of
medications for opioid use disorder, including methadone. Results obtained from steps
one and two above were verified against results from the ASAM report to ensure that no
state Medicaid programs were incorrectly omitted that had been identified as providing
coverage for methadone in 2013.
Step four. The Kaiser Family Foundation (KFF) produces reports based on surveys of
state Medicaid programs. We used their publicly available fiscal year (FY) 2017 survey
information to verify our results on methadone. This information is summarized in their
State Health Facts (KFF, 2018), and additional information is available in the report
titled Medicaid Moving Ahead in Uncertain Times (KFF, 2017). If the KFF information
indicated that methadone was not covered at the point of its survey but we located
information to the contrary on the state program website, we included the state as
covering methadone for MAT (Alabama, Indiana, and Iowa). If the KFF information
indicated that methadone was covered, we counted it as covered, even if we found no
evidence on the state Medicaid program website or elsewhere (Alaska, Kansas,
Mississippi, Missouri, Montana, Oklahoma, and South Dakota).
Determination of preferred status and benefit limits. If it was determined that methadone
was reimbursed as MAT, the process listed below was followed to ascertain applicable benefit
design limits.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
31
Step one. If behavioral health or other materials clearly indicated that methadone benefit
limits such as prior authorization applied to the administration of methadone for MAT,
those materials were used to define status and limits.
Step two. In all other instances, the status, prior authorization, quantity limits, or other
benefit limits that applied to methadone were treated as not applying to methadone
provided for MAT.
Step three. The 2013 ASAM report utilized a blanket rule that prior authorization
requirements do not apply to methadone. This approach was accepted unless it was very
clear, pursuant to the research steps outlined above, that prior authorization was required
by a state program for methadone maintenance and not for pain.
14
In addition to any state requirements or benefit limits that precede or accompany methadone
administration, providers also must comply with federal regulations that govern access to
methadone. These regulations do not impose restrictions directly related to reimbursement
but, instead, are designed to enhance safety and efficacy and to reduce the possibility of
diversion. Although they are not reimbursement-related, the federal regulations arguably
affect access to methadone as MAT in ways that are similar to some state Medicaid
requirements for reimbursement of other MAT medications. One example includes a
requirement that patients younger than 18 years of age have had two documented
unsuccessful attempts at short-term detoxification or drug-free treatment within a 12-month
period to be eligible for methadone maintenance treatment. Although it is not linked to
reimbursement in any way, this universal federal requirement (42 CFR § 8.12(e)(2)) arguably
has a similar effect on access, as does step therapy. Some other requirements found at 42
CFR § 8.12 (Federal Opioid Treatment Standards, 2002) include the following, which must
be satisfied to allow methadone administration:
o Requirements for evaluation and assessment, including diagnostic requirements
o Additional age restrictions
o Requirements related to pregnancy
o Requirements related to number of detoxification attempts and length of
dependency
o Requirements for provision of medical, counseling, vocational, educational, and
other assessment and treatment services
o Requirements for drug testing
o Requirements that methadone be administered orally
o Requirements regarding take-home doses, including limitations on such dosing
14
Please see discussion of results related to methadone for an explanation of the very limited circumstances in
which prior authorization or quantity limits may apply to the administration of methadone in MAT.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
32
These regulations are clarified further in SAMHSA’s March 2015 Federal Guidelines for Opioid
Treatment Programs (SAMHSA, 2015). Because these are not reimbursement-related, they are
not included in our findings related to methadone in this report.
Moreover, although some states directly incorporate aspects of the federal regulations into their
state Medicaid policies (e.g., Massachusetts regarding age [Massachusetts Department of Public
Health,, 2016]) or specify that methadone treatment may occur only through a federally certified
OTP (e.g., Vermont [Department of Vermont Health Access, 2017]), they also were not noted as
state-specific requirements in the analysis because these state reimbursement-related restrictions
do not differ from the federal treatment standards.
Methodology Limitations
Potential limitations to our approach for classifying drugs and state Medicaid benefit limitations
include the following: (1) reliance on publicly accessible documents that did not include all
Medicaid MCO documents, which means that there may be additional or inconsistent benefit
restrictions placed by some MCOs that were not captured, and (2) the fact that some Medicaid
Section 1115 waivers may limit coverage to parts of states, so certain areas may have more
limited funding for some types of MAT. A further limitation of this study is that methadone is
reimbursed by states in different ways depending on whether it is used for MAT or for pain, but
this report only focuses on reimbursement for MAT. Methadone used as a treatment for pain is
reimbursed similarly to other on-MAT pharmaceutical options. However, given that methadone
is involved in one out of every three accidental overdose deaths, it has been suggested that the
process for obtaining methadone to treat pain might also benefit from greater controls (Vestal,
2015). An expanded analysis of the financing policies for methadone could take into
consideration the fact that adverse outcomes may be associated with its use for the treatment of
pain in addition to MAT and expand the scope of the financing policies included in the analysis.
Medicaid Benefit Limits on Medications for Alcohol and Opioid Use
Disorders
State Medicaid programs use various techniques in their benefit designs to try to constrain costs
and encourage the proper use of medications for alcohol and opioid use disorders. In general,
more management techniques were used for medications to treat opioid use disorders than those
to treat alcohol use disorders. The findings for the different types of benefit limits for opioid and
alcohol MAT are discussed below, as are separate findings for methadone and naloxone.
15
Detailed information regarding each drug may be found in the Appendix A tables.
15
Our discussion here excludes the U.S. Virgin Islands because of a lack of pertinent Medicaid coverage
information.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
33
States may reimburse for medications regardless of preferred status. Examination of
reimbursement showed that Medicaid coverage of alcohol and opioid use disorder medication
treatments varies. As shown in Figure 1, some states show no evidence of covering disulfiram (2
states), acamprosate (11 states), implantable buprenorphine (14 states), or extended-release
injectable buprenorphine (18 states). Other than these four drugs and methadone, which is not
covered by Medicaid in nine states, state Medicaid plans cover all other SUD medications.
16
In
every state, some form of naloxone is reimbursed—Evzio is least likely to be covered. Puerto
Rico covers buprenorphine and buprenorphine-naloxone, but coverage for other alcohol or
opioid treatments is uncertain.
16
For additional information on Medicaid coverage of buprenorphine, please see the Medicaid Coverage of
Effective Treatment for Opioid Use Disorder (Urban Institute, 2017).
Benefit Design Elements
Common Medicaid benefit design elements and their respective definitions are listed below.
Preferred Drug List (PDL) is a list of the drugs that providers usually are permitted to prescribe
without seeking prior authorization. If a drug is not included on the PDL, the provider may need to
obtain approval from the state Medicaid agency before the drug can be dispensed. Not every state
Medicaid agency has a PDL, and some drugs on a PDL may still require prior authorization.
Prior Authorization is a broad term that requires a prescriber to obtain permission from the
pharmacy benefit plan prior to prescribing a product to a member. Without permission, the product
will not be covered. Some prior authorization forms require the prescribing medical provider to
have referred the patient to concurrent behavioral therapy, for the patient to have a negative urine
drug screen or documented adherence to a treatment plan, or other requirements. Such
requirements also may be imposed by payers through clinical edits. Clinical (point-of-sale) edits is
a generic term for a variety of reviews that are conducted, often at the benefit plan and pharmacy
levels, to help ensure the therapeutically prudent use of pharmaceutical products as well as the
optimization of benefit program funds. With drug utilization reviews, claims processors match
information on claims against a clinical database and a member’s prior pharmacy history to assess
clinical issues with prescriptions, duplication of therapy, and compatibility. These reviews can stop
prescriptions from being filled until the prescriber corrects a discrepancy, or they will prompt a
warning to the pharmacist before dispensing.
Psychosocial treatment includes counseling and other nonmedication interventions that may be
required to obtain reimbursement for a medication as part of MAT.
Quantity level limits define the maximum quantity of medication that is covered for one
prescription or copayment. Typically, a prescription may be for only a 30-day supply or (in the case
of mail-order prescription) a 90-day supply.
Step therapy occurs when a claims processor must verify that the patient first tried a more cost-
effective medication before filling a more expensive alternative. If the first-line treatment was
ineffective or not tolerated by the patient, the “next step up” may be authorized.
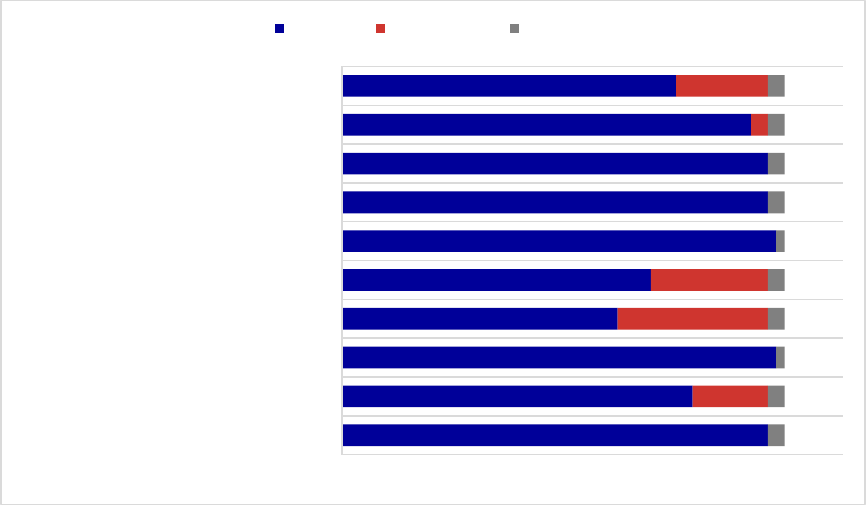
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
34
Figure 1. Medicaid Coverage of Medications for Alcohol and Opioid Use Disorders
or to Reverse Opioid Overdose, 2018
a
Abbreviation: ER, extended-release.
a
Materials reviewed were those available in the second and third quarters of 2018.
Sources: State documents listed in Appendix A, Table A-1, the 2018 Medicaid State Drug Utilization data.
To ascertain preferred status, researchers assessed whether at least one version of each drug has
preferred status by at least one Medicaid plan per state (Figure 2). For medications specific to
alcohol use disorder treatment only, 32 states and 27 states afforded disulfiram and acamprosate
preferred status, respectively. Oral naltrexone, which may be used for the treatment of either
alcohol or opioid use disorder, has preferred status in 44 states, and extended-release injectable
naltrexone is preferred in 34 states, perhaps indicating a decision on the basis of cost.
Buprenorphine is preferred in only 29 states, and even those states typically limit its use with
preferred status to pregnant women or other clinically limited situations. In contrast,
buprenorphine-naloxone is preferred in 51 states. Preferred status varies considerably, however,
for buprenorphine-naloxone by formulation across states, with preferred status sometimes given
to the generic or to one or more of Suboxone, Suboxone film, Zubsolv, or Bunavail, depending
on the state. Forty-three states treat some form of naloxone as preferred. Attributing preferred
status to methadone as a form of MAT is not possible, given the different method of dispensing
and strict restrictions on access governed by federal law.
51
42
52
33
37
52
51
51
49
40
9
18
14
2
11
2
2
1
2
2
1
2
2
2
2
NALOXONE
METHADONE
BUPRENORPHINE- NALOXONE
ER-BUPRERORPHINE
IMPLANTABLE BUPRENORPHINE
BUPRENORPHINE
ER-NALTREXONE
ORAL NALTREXONE
DISULFIRAM
ACAMPROSATE
NUMBER OF MEDICAID PROGRAMS
Covered Not Covered Unknown
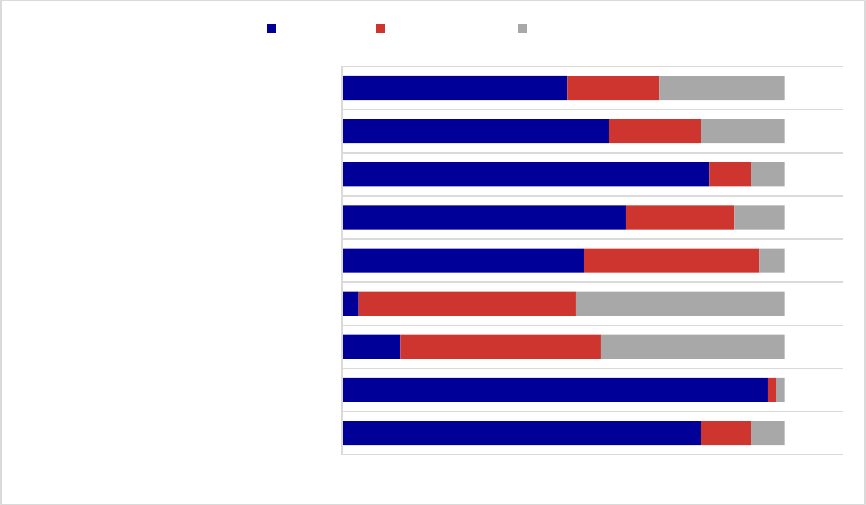
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
35
Figure 2. Preferred Status of Medications for Alcohol and Opioid Use Disorders or
to Reverse Opioid Overdose, 2018
a
Abbreviation: ER, extended-release.
a
Materials reviewed were those available in the second and third quarters of 2018.
Sources: State documents listed in Appendix A, Table A-1.
Prior authorization is frequently required in Medicaid programs for medications to treat opioid
and alcohol use disorders, although recent policies have led to changes in this area. Examples
include new Section 1115 waivers, such as in Virginia, where prior authorization for some drugs
is explicitly being eliminated, and steps being taken by certain health plans to eliminate prior
authorization requirements for some or all MAT. Because prior authorization requirements often
are inconsistent across a state’s Medicaid FFS plan and different Medicaid MCOs, in this report,
a state was counted as having a prior authorization requirement for a medication if any one of the
FFS or selected MCO plans required it for the generic version of the drug even though just
because one plan has such a policy does not indicate that this is the policy of that state’s
Medicaid program. If only a brand medication is available in that state, our analysis of prior
authorization only is applied to reimbursement for the brand drug. Among the Medicaid
programs with available information on their pharmacy benefit management designs, prior
authorization requirements for buprenorphine and buprenorphine-naloxone were most common
(Figure 3), required by 40 and 31 Medicaid programs, respectively, with prior authorization
requirements for buprenorphine-naloxone varying greatly by formulation across states.
Authorization for use of buprenorphine is available most often for pregnant women and less so
for those who are breastfeeding or who have a documented intolerance to naloxone that may
interfere with use of the buprenorphine-naloxone combination. Clear prior authorization
requirements for implantable buprenorphine and extended-release injectable buprenorphine were
seen in 26 and 25 states, respectively, although many states do not include these medications in
43
51
7
2
29
34
44
32
27
6
1
24
26
21
13
5
11
11
4
1
22
25
3
6
4
10
15
NALOXONE
BUPRENORPHINE- NALOXONE
ER-BUPRENORPHINE
IMPLANTABLE BUPRENORPHINE
BUPRENORPHINE
ER-NALTREXONE
ORAL NALTREXONE
DISULFIRAM
ACAMPROSATE
NUMBER OF MEDICAID PROGRAMS
Preferred Not Preferred Unknown
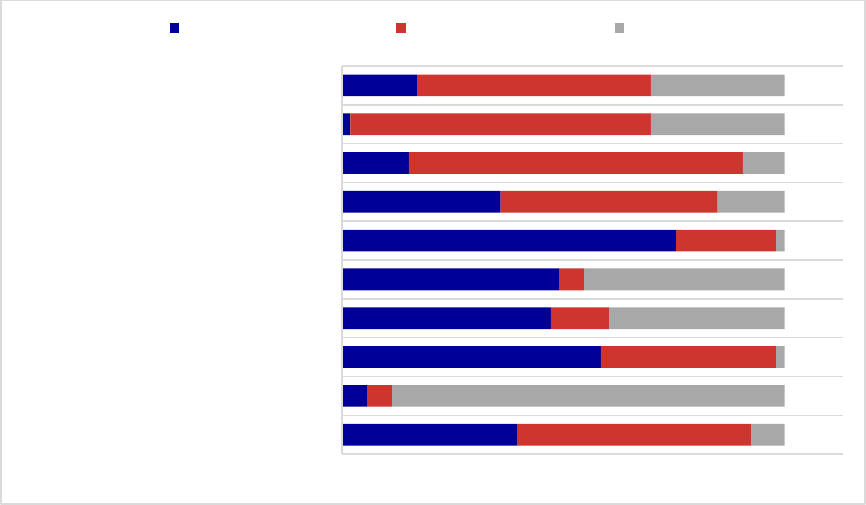
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
36
their pharmacy benefits, treating them, instead, as medical benefits with different criteria.
Among the naloxone formulations, Evzio is most likely to require a prior authorization.
Figure 3. Prior Authorization Requirements for Medications for Alcohol and
Opioid Use Disorders or to Reverse Opioid Overdose, 2018
a
Abbreviation: ER, extended-release.
a
Materials reviewed were those available in the second and third quarters of 2018.
Sources: State documents listed in Appendix A, Table A-1.
Prior authorization for oral naltrexone was required in 8 states and, for extended-release
injectable naltrexone, in 19 states (see Figure 4). In general, fewer programs required prior
authorization for disulfiram (one state) and acamprosate (nine states). In Figure 4, we illustrate
the distribution of prior authorization requirements for extended-release injectable naltrexone
across the U.S.
21
3
31
25
26
40
19
8
1
9
28
3
21
7
3
12
26
40
36
28
4
47
1
21
24
1
8
5
16
16
NALOXONE
METHADONE
BUPRENORPHINE- NALOXONE
ER-BUPRENORPHINE
IMPLANTABLE BUPRENORPHINE
BUPRENORPHINE
ER-NALTREXONE
ORAL NALTREXONE
DISULFIRAM
ACAMPROSATE
NUMBER OF MEDICAID PROGRAMS
Yes Prior Authorization No Prior Authorization Unknown
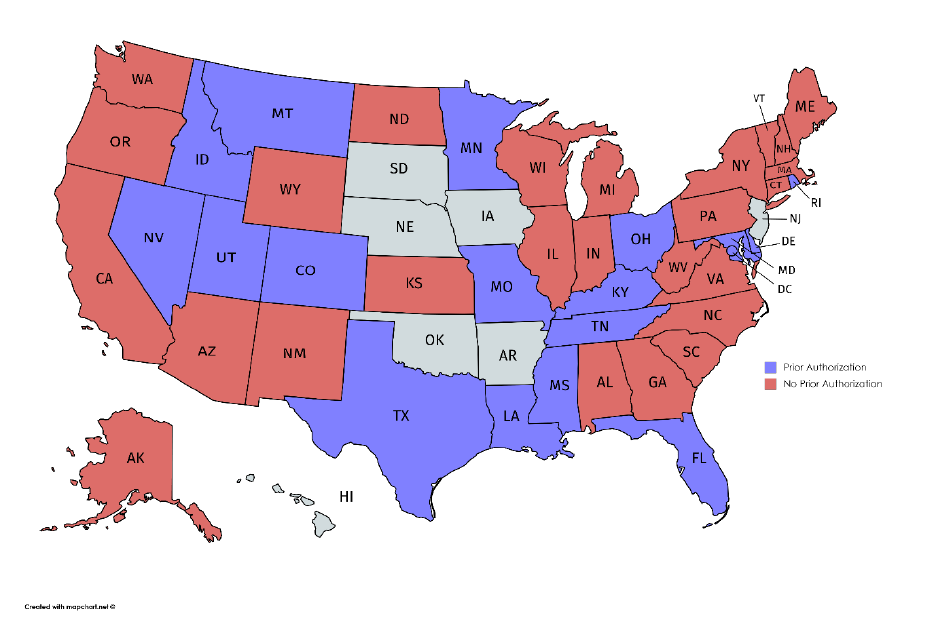
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
37
Figure 4. Prior Authorization Requirements for Extended-Release Injectable Naltrexone,
2018
a
a
Materials reviewed were those available in the third quarter of 2018.
Sources: State documents listed in Appendix A, Table A-1; Table A-6.
Several states also required evidence that the patient was being referred to or receiving
psychosocial treatment with his or her medications. These requirements almost exclusively
applied to medications for opioid use disorders. Documentation of psychosocial treatment was
required by 16 programs for buprenorphine and for buprenorphine-naloxone, 10 programs for
extended-release injectable buprenorphine, 5 programs for implantable buprenorphine and
extended-release injectable naltrexone, 3 programs for acamprosate, 1 program for oral
naltrexone, and no programs for disulfiram. Two states required receipt of psychosocial
treatment to receive some forms of naloxone.
Quantity or dosing limits often are used by Medicaid programs (Figure 5). Quantity or dosing
limits are least common for disulfiram (3 states), oral naltrexone (5 states), acamprosate (12
states), and extended-release injectable naltrexone (16 states). In contrast, quantity or dosing
limits for buprenorphine and buprenorphine-naloxone were used by 45 and 46 states,
respectively.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
38
Figure 5. Quantity or Dosing Limit Requirements for Medications for Alcohol and
Opioid Use Disorders or to Reverse Opioid Overdose, 2018
a
a
Materials reviewed were those available in the second and third quarters of 2018.
Sources: State documents listed in Appendix A, Table A-1.
In addition to quantity limits, some states have established lifetime treatment limits, most
commonly applied to buprenorphine and buprenorphine-naloxone. Along with other changes in
response to the opioid crisis and, to some extent, possibly parity implementation, these limits
have begun to disappear. This study found, in mid-2018, only one instance of a state imposing a
lifetime limit. Specifically, New York regards any treatment using implantable buprenorphine
for longer than 1 year as “investigational” and not medically necessary. A pharmacy benefit
management strategy of imposing a lifetime limit is one that payers should consider carefully
because it can impose significant challenges for individuals with opioid use disorders. Both
opioid and alcohol addiction are chronic diseases; consequently, such limitations in MAT may
increase the number of individuals who are at risk for relapse even after long periods of
abstinence (McLellan, Lewis, O’Brien, & Kleber, 2000; NIDA, 2012c).
Step therapy also is required in some programs for certain medications. Often this is a
requirement that the generic drug be tried unsuccessfully before a brand or that a preferred drug
be tried unsuccessfully before a nonpreferred drug. Some indications of step therapy
requirements are found in MCO documents without clear indication as to the nature of the
progression required. Some sort of step therapy on disulfiram and acamprosate is imposed in
25
1
46
17
13
45
16
5
3
12
8
6
3
4
7
15
33
25
17
20
52
1
33
36
1
22
15
25
24
NALOXONE
METHADONE
BUPRENORPHINE- NALOXONE
ER-BUPRENORPHINE
IMPLANTABLE BUPRENORPHINE
BUPRENORPHINE
ER-NALTREXONE
ORAL NALTREXONE
DISULFIRAM
ACAMPROSATE
NUMBER OF MEDICAID PROGRAMS
Limits No Limits Unknown

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
39
one state and four states, respectively. One state requires step therapy for oral naltrexone, and
four impose it on extended-release injectable naltrexone. Step therapy for extended-release
injectable naltrexone likely is required because it is a relatively expensive medication, and in
some states, buprenorphine drugs are preferred for treatment of opioid use disorder. Step therapy
requirements are more common for the buprenorphine drugs. Nine states require it for
buprenorphine, and most require a showing of serious documented adverse reactions to
naloxone. At least nine states require step therapy for
implantable buprenorphine and three for extended-release
injectable buprenorphine. Two states apply step therapy to
buprenorphine-naloxone, although the formulations for
which it is required (e.g., Bunavail, Zubsolv) vary by
state. Two states require step therapy using other forms of
naloxone before they will reimburse Evzio.
Methadone is covered for MAT by Medicaid in 42 of 53
states and territories.
17
Methadone as MAT, in contrast to
pain treatment, is subject to a unique federal regulatory
structure that governs administration of and access to the
drug. The federal regulations impose restrictions such as
required counseling, observed dosing requirements, and
that those under 18 years of age have met certain criteria
before methadone may be administered. Additionally,
because methadone for MAT is not covered under
pharmaceutical benefits but as a medical service,
pharmaceutical benefit limitations generally do not apply.
There are, however, a few states that clearly have
Medicaid reimbursement limits that apply to the service of
methadone maintenance and that, consequently, affect
access to the medication. These state Medicaid limits
include those in three states (Connecticut, Maine, and
North Carolina) that require prior authorization for
methadone maintenance.
Naloxone is not technically MAT but is an opioid overdose treatment that comes in three
formulations. All 50 states and the District of Columbia cover at least one of those formulations,
23 cover all three, and 28 states do not cover Evzio (auto-injector). Forty-three states identify at
least one formulation as preferred, with Evzio least likely to be preferred and most likely to
require prior authorization. Two states require documentation of counseling, and that is only
17
For Alaska, Kansas, Mississippi, Missouri, Montana, Oklahoma, and South Dakota, we found no evidence of
methadone coverage in state Medicaid or MCO documents. We relied on KFF documents based on surveys of state
Medicaid programs to identify methadone MAT coverage in these states (KFF, 2017, 2018).
Substance Use Disorder
Relapse
Substance abuse treatment,
including medication therapy,
enables people to counteract
the disruptive effects of
addiction on the brain and
behavior and to regain control of
their lives. However, the
chronic nature of the disease
means that relapse is likely.
Studies have found that 1 year
after discharge from treatment
programs, 40 percent to 60
percent of individuals relapse in
using alcohol or illicit drugs
(McLellan, Lewis, O’Brien, &
Kleber, 2000). This finding is
similar to the 30 percent to 50
percent relapse rate for adults
with diabetes and to the 50
percent to 70 percent relapse
rate of adults being treated for
hypertension or asthma
(McLellan et al., 2000).
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
40
when reimbursement is sought for Evzio (two states) or Narcan (one state). Quantity limits vary
greatly by formulation and by state. Only two states require step therapy for naloxone—New
York and South Carolina (both for Evzio)—which would require problematic use of the
preferred version of the drug before the nonpreferred version may be reimbursed.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
41
IV. Innovative MAT Provision, Coverage, and Financing
Models
This section describes examples of innovative models across the states for achieving effective
results in the provision of MAT and other treatment of opioid and alcohol use disorders. It also
highlights some cross-cutting best practices that may help to increase access to SUD
medications.
A. Innovative Models
Three approaches to the provision and financing of treatment that include MAT were identified
for discussion.
18
These include the Massachusetts Nurse Care Management Model, a model that
also was discussed in the first edition of this report published in 2014; statewide integration of
MAT in state-contracted SUD treatment in Missouri; and innovative MAT approaches in
Washington, including a telemedicine MAT pilot project. Potential approaches were identified
through initial interviews with staff of the American Society of Addiction Medicine, the National
Association of Medicaid Directors, the National Association of State Alcohol and Drug Abuse
Directors, and the National Association of State Mental Health Program Directors. Based upon
those interviews and on a review of public sources, the authors of this report also conducted
semi-structured telephone interviews with state or program staff to obtain further information.
Detailed notes were taken on those interviews, summaries were prepared in the form of case
studies and the state and program staff were provided an opportunity to review the summaries for
accuracy and completeness.
Nurse Care Management Model, Massachusetts
The Massachusetts Nurse Care Management Model of treatment for SUDs was developed to
respond to a shortage of clinical support for outpatient buprenorphine treatment. Nurse care
managers (NCMs) are an integral part of the Massachusetts Collaborative Care Model of office-
based opioid treatment with buprenorphine (OBOT-B). The model allows waivered physicians
more time to manage a larger group of patients by having NCMs work with program
coordinators or medical assistants to perform much of the initial assessment, education
management, referral to addiction treatment, adherence monitoring, admission paperwork, and
communication with prescribing physicians, addiction counselors, and pharmacists. The
program allows the NCMs to have daily interaction, either in person or by phone, with each
patient receiving buprenorphine (Alford et al., 2011; LaBelle, Han, Bergerson, & Samet, 2016).
The model, which adheres to recognized practice standards, includes four treatment stages: (1)
18
For more comprehensive information about MAT treatment models that focuses on provision of MAT in primary
care, the reader is pointed to the publication Medication-Assisted Treatment Models of Care for Opioid Use
Disorder in Primary Care Settings (Agency for Healthcare Research and Quality, 2016).

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
42
screening and assessment of appropriateness for OBOT-B, (2) medication induction, (3)
stabilization, and (4) maintenance (LaBelle et al., 2016).
The pilot project began in 2003 at the Boston Medical Center. After a successful demonstration
in that setting, it was expanded to community health centers (CHCs) throughout the state. The
project was initially funded for up to 7 years by the Commonwealth of Massachusetts, with block
grant funding paying for nonbillable services,
including the salary of a full-time registered
nurse to allow the provision of nonbillable
services, staff training, and technical support on
implementation of protocols for each program.
An initial cost study of 16 CHCs determined that
the NCMs needed to manage a caseload of 100
patients to cover their salaries. This was
achievable in most of the CHC participating
programs, and the expectation subsequently was
increased to 125 patients per NCM, with the
assistance of a medical assistant (LaBelle et al.,
2016).
The state OBOT-B program director also provides training and technical assistance (TA) to the
CHCs. For example, NCMs are provided with 8 hours of buprenorphine training that is modeled
on the required waiver training for physicians while being modified to fit the needs of the
program, with quarterly follow-up training (LaBelle et al., 2016). On-site and remote training,
TA, and clinical supervision allow additional training for other CHC personnel involved in
MAT. The provision of clinical support to physicians and the provision of training and TA to the
NCMs are critical elements for the success of the program (LaBelle et al., 2016).
As the program matured, it went through some notable changes. First, the title of the program
has changed from Office-Based Opioid Treatment (OBOT) to Office-Based Addiction Treatment
(OBAT) to provide a better explanation of the scope of the model. This makes clear that the
program addresses all SUDs rather than only opioid disorders and that is does not rely
exclusively on medication but incorporates other treatments and supports for patients, including
for those who may be in remission. Second, extended-release injectable naltrexone increasingly
is used to treat both opioid and alcohol use disorders, supplementing buprenorphine and other
older medications in the treatment arsenal. Third, the model incorporates risk reduction to keep
patients engaged in treatment even when they are struggling. This recognizes the profound risk
that accompanies nontreatment for those who may not be capable of abstinence. Fourth, Boston
Medical Center is participating as faculty in the national Project Extension for Community
Healthcare Outcomes (ECHO) Opioid Addiction Treatment, which provides training and
technical assistance in addiction treatment to providers in clinics around the country, including
rural and underserved communities (UNM School of Medicine, 2017).
OBAT is the New OBOT
The Office-Based Opioid Treatment
(OBOT) model is now the Office-Based
Addiction Treatment (OBAT) model. This
change recognizes that—
• MAT and the office-based model
address alcohol and other drug
dependence besides opioid
dependence
• The model does not rely only on
medication but incorporates other
treatments and patient supports

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
43
Funding also has evolved as the Massachusetts NCM program has grown. Many of the CHCs
are federally qualified health centers (FQHCs), which use a Medicaid prospective payment
system model that is a capitated approach allowing flexibility for services rendered. More recent
cost modeling data for the FQHCs show that, if an NCM manages a caseload of 40 patients and
sees each of them 27 times a year, the prospective payment system will cover salary,
administrative costs, and fringe benefits. NCMs working in the model presently manage 100 to
125 patients, far more than are needed to break even. Consequently, several CHCs have hired
additional nurses to reduce the caseload. Not all CHCs can use this funding model, however,
because they do not have the FQHC recognition. Other supplemental funding sources include
general state funding, federal Health Resources and Services Administration funding, SAMHSA
grants, and funding from a large Boston-area employer (LaBelle et al., 2016).
Statewide Integration of MAT into SUD
Treatment, Missouri
The Missouri Division of Behavioral Health
works to integrate MAT into all SUD treatment
in the state, requiring any SUD treatment
provider who contracts with the Division to
offer MAT either directly or by formal
arrangement. Prior to 2006, most MAT in
Missouri involved methadone; nonmethadone
treatment providers had not successfully
integrated other forms of MAT such as
naltrexone. Beginning in 2006, the state
recruited a group of volunteer providers to
participate as part of a Robert Wood Johnson
Foundation Advancing Recovery grant, in part
to identify barriers and opportunities to the use
of oral naltrexone and acamprosate for alcohol
use disorders. From this experience, the state developed an expectation that all contracted
providers would integrate MAT into their service delivery starting in 2007. In 2008, Missouri
was one of the earliest advocates for making Vivitrol available in treatment. The use of this
medication expanded beyond the community in 2012 when the St. Louis City Drug court began
using Vivitrol prior to participant release from jail. This idea was adapted to the state
correctional system with a pilot program that offered Vivitrol to prison inmates nearing release,
with continuing provision of MAT in the postrelease period. The successful integration of MAT
into SUD and co-occurring disorders treatment led the state to require all Certified Community
Behavioral Health Clinics in the Prospective Payment System demonstration provide all forms of
MAT other than methadone.
Provider Capacity Issues
Missouri has undertaken several
approaches to addressing provider
capacity issues:
• Telehealth
• Contracting with primary care
providers for MAT
• Contracting with OTPs for
prescribers
• Expanding OTP prescribing beyond
methadone
• Relying on nurse practitioners to use
naltrexone and acamprosate, leaving
waivered physicians more time to
prescribe buprenorphine
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
44
Because Missouri state government has worked to implement MAT for at least a decade, it has
been in the position to recognize and address several challenges:
Expanding capacity to prescribe buprenorphine in Missouri has been slow because of a
shortage of prescribers. Some interim solutions have included use of telehealth, first
available in 2009, and contracting for prescribing services from local primary care
providers and OTPs. Rather than relying solely on methadone, OTPs also are expanding
their menu of medications to include buprenorphine, naltrexone, and other drugs.
Although the state is working to expand access to waivered providers through training
and education/awareness efforts, the new federal law that expanded buprenorphine
prescribing privileges to nurse practitioners and physician assistants may be impeded by
strict state licensing regulations. To circumvent that, nurse practitioners are sometimes
used to administer Vivitrol, whereas waivered physicians manage buprenorphine clients.
Like many other states, there is some controversy about treating SUDs with medications.
Education and the growing scientific evidence that supports efficacy has helped change
provider, prescriber, consumer, and family attitudes.
Missouri has learned that establishing equitable reimbursement that covers medications,
medication administration, laboratory services, other related activities, and administrative
overhead is important to ensure that providers are fairly compensated for the provision of
MAT.
Missouri also has been at the forefront of integrating behavioral health treatment, including
MAT, into primary care. This has involved different strategies, including hiring physicians to
staff the SUD agency, establishing relationships with FQHCs, co-locating SUD and FQHC
services, and contracting with community providers. The use of telehealth has further facilitated
the integration of SUD treatment, including MAT, into primary care. Challenges that the state
has confronted include the need for primary care provider education related to the treatment of
SUDs. Missouri also has found that addressing negative perceptions of SUD treatment, whether
held by providers, patients, or patients’ families, is critical to success.
Missouri has found ways to address barriers that often are encountered, whether MAT is
provided by a specialty SUD provider or by a primary care physician. These include the
following:
Supporting champions at both the state and provider level, specifically individuals who
can serve as leaders in moving forward with the delivery of MAT, including doctors who
can serve as mentors and trainers for other providers.
Monitoring prescribing patterns within organizations to ensure that prescribers are
providing patient-centered care, rather than prescribing particular medications uniformly
regardless of individual need. This has been facilitated by a robust health information
and data system that is already well established.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
45
Continuing efforts to adopt prescribing protocols that make use of the existing evidence
base in a way that makes sense within the Missouri system of treatment.
Flex Care Telemedicine-MAT Pilot Project and Other Innovative MAT Approaches,
Washington
Using a 2015 SAMHSA Medication-Assisted-Treatment Prescription Drug and Opioid
Addiction (MAT-PDOA)
19
grant to the state of Washington, Evergreen Treatment Services
implemented a Flex Care pilot telemedicine project at the Grays Harbor Clinic in the township of
Hoquiam. Hoquiam is located in a rural area on
the central Washington coast about 2 hours
southeast of Seattle, and many people in that part
of the state were unable to access MAT prior to
implementation of the Flex Care project.
Although some patients still travel up to 3 hours
to attend the Grays Harbor Clinic, the
introduction of telemedicine has made a
difference for many individuals struggling with
opioid use disorder in isolated areas of the
Washington coast. The success of this pilot will determine whether the state expands to other
settings, like to Grays Harbor Clinic, that are not FQHCs.
The Grays Harbor Clinic opened as an OTP in 2014. Upon receipt of the MAT-PDOA award, it
was difficult to find a waivered physician to prescribe Suboxone because of the isolated location
and stigma within the medical community related to the patient population. To resolve this
problem, Evergreen implemented a practice incorporating MAT, telemedicine, and two proven
models of care. The first model was the Massachusetts OBOT-B model, also known as the
Nurse Care Manager model, in which an NCM provides most medical visits with a patient and
coordinates and manages integrated treatment with other providers, as needed. The second
model was The Johns Hopkins School of Medicine Collaborative Opioid Prescribing model of
OTP/OBOT coordinated care, which provides flexibility as to the level of care at which the
patient is treated on the basis of patient need. This hybrid approach has allowed the clinic to
provide services to a larger group of patients, many of whom previously could not access care.
To implement telemedicine, Evergreen contracted with a physician in Portland, Oregon. The
physician provides prescribing oversight while an on-site staff member is with the patient during
the telemedicine session and assists with the telemedicine equipment. The project initially relied
19
More information on the MAT-PDOA is available at Substance Abuse and Mental Health Services
Administration. (2018). State grant programs. Retrieved from
https://www.samhsa.gov/medication-assisted-
treatment/mat-pdoa
Expanding Access to MAT
via Telemedicine
Approximately 200 patients in rural
coastal Washington state who had no
access to medication assisted treatment
for their opioid dependence disorder, now
receive MAT as part of the Washington
state Flex Care treatment model.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
46
on the NCM to manage the telemedicine equipment but has now moved to using medical
assistants for that role. The NCM manages most of the rest of the patient’s visits. Patients come
in weekly for a check-in with the NCM and to receive a prescription for Suboxone. Depending
on how a patient progresses, he or she transitions to biweekly then to monthly or quarterly visits
with the NCM. The off-site physician will conduct a telemedicine session with the patient at
least quarterly. Incorporation of the Collaborative Opioid Prescribing model allows for unstable
patients to be admitted to the OTP and then to move into maintaining their care with the Flex
Care team once they are stabilized on the medication regime. Because the model incorporates a
continuum of care, however, it allows the patient to return to the OTP for treatment if he or she
destabilizes, resuming treatment by the Flex Care medical staff once again after restabilization
occurs. This model has provided motivation for other primary care providers in the area to
participate in the program, prescribing and overseeing Suboxone once patients are stabilized,
because there is a reliable back-up specialty provider to step in if a patient destabilizes or
becomes too challenging for the community physician to manage.
Program staff report that the Flex Care program has encountered several challenges, some of
which have been resolved and some of which are ongoing.
Volume. The pilot program cannot see as many patients via telemedicine as originally
anticipated because of the need to have a staff person in the room as well as the off-site
physician in place. This is an on-going concern that affects the level of reimbursement
available to the clinic.
Cost. The telemedicine equipment costs approximately $30,000 per unit. Although the
clinic’s unit was paid for by the MAT-PDOA grant, this cost could deter other nonprofits
from adopting telehealth absent supplemental grants or other funding. Reimbursement
and staffing are other essential components of cost.
Equipment management. The clinic had to hire additional staff to manage the
telemedicine equipment during the sessions with the doctor. It is a task that can be
performed by someone who is paid less than a nurse and is now performed by a medical
assistant, but it was an unanticipated cost that contributes to the unsustainability of the
program unless reimbursements are addressed.
Staffing. In addition to hiring staff to manage the equipment, the clinic has had difficulty
finding nursing staff in rural locations. There is a nursing shortage in the area, and the
clinic lost the nurse care manager who started the program. The clinic currently is using
a more expensive advanced nurse practitioner and is paying for additional physician time
to compensate. The clinic is hoping to attract qualified personnel to replace the original
nurse care manager.
Infrastructure. Connectivity between the rural location and the remote physician was
initially difficult and highlighted technical issues that had to be resolved, leading to
improved information technology capabilities for the clinic.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
47
Sustainability. The grant will provide funding until July 2018. The clinic is exploring
other avenues of reimbursement to cover costs when the grant ends. Historically, the
agency had not used insurance contracts and began the process of contracting with payers
to initiate that reimbursement opportunity. For Medicaid patients, the level of
reimbursement alone may not cover the cost of operating the telemedicine program,
possibly requiring additional funding sources.
Access to treatment for patients in rural coastal Washington has improved because of this
innovative approach to the provision of MAT. Approximately 200 patients now receive MAT
who could not access any care before this program was implemented. This is the only option
available to obtain MAT for these patients. To continue availability for those patients, the Grays
Harbor Clinic is exploring options to sustain the Flex Care program of treatment for those with
opioid use disorders.
In addition to the Flex Care telemedicine model being implemented at Grays Harbor Clinic, there
are several other innovative approaches to MAT in Washington. These include the following:
Use of mobile methadone vans to reach patients
A program in Port Angeles inducting county jail inmates with buprenorphine and
transitioning them into a nearby community health center for follow-up (Port Angeles is
located about 3 hours northwest of Seattle).
A citywide emergency department multidisciplinary care-coordination program in
Spokane in which all EDs in the area are connected to a web-based information exchange
system (Murphy & Neven, 2014)
A Seattle-based buprenorphine induction program affiliated with a needle exchange
program, in which patients subsequently are transitioned to community providers.
B. Cross-Cutting Best Practices
Three factors that may affect access to MAT include lifetime limits, level of burden associated
with prior authorization, and state licensure requirements. Each is addressed below.
Historically, a number of Medicaid programs imposed lifetime limits on the use of medications
for alcohol or opioid use disorders. Eleven states had lifetime limits on buprenorphine-
naloxone when the first edition of this report was published in 2014. Currently, however, only
one state imposes such a limit on implantable buprenorphine, and that is on the grounds that
longer use is considered “investigational.” SUD is a chronic, relapsing disease similar to
diabetes or asthma, and a lifetime limit for opioid treatment is inconsistent with evidence and
best practices. For example, a randomized multisite study found that stopping buprenorphine-
naloxone after a 12-week treatment course resulted in a rate of unsuccessful outcomes that
exceeded 90 percent (Weiss et al., 2011). The decrease in lifetime limits may relate to parity
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
48
or reflect the realities of addressing the opioid crisis, but represents progress in assuring access
to treatment.
Prior authorization is a common benefit
design element, particularly for
buprenorphine and buprenorphine-
naloxone. The prior authorization and
subsequent reauthorizations of
buprenorphine and buprenorphine-
naloxone may require various types of
documentation. Some states require
more extensive documentation than
others. These requirements may include
documentation of the patient’s opioid
addiction, drug screening tests,
supplemental psychosocial treatment,
tapering plans, or a specific plan for
following up with the patient (e.g.,
frequency of office visits) (see Appendix
A, additional specifications to Tables A-
7, A-8, A-9, and A-10 for some of the
prior authorization requirements).
Although these requirements aim to
ensure the proper use of buprenorphine
and buprenorphine-naloxone, they may
delay access to treatment and add
significant provider burden. The end result is often that the patient does not receive any
medication (ASAM, 2013). Standard operating rules concerning prior authorization for all
medications, including buprenorphine, should improve access to medications used to treat
alcohol and opioid use disorders. Increasingly, payers are moving toward a system of real-time,
electronic prior authorization at the point of care that may reduce interruptions to patient flow or
access to medications (covermymeds®, 2017). Providers and pharmacies such as CVS
Caremark (http://www2.caremark.com/epa) also are moving toward this electronic system
facilitating authorization.
State licensure requirements affect whether a nurse practitioner or other nonphysician provider
may legally prescribe, including whether they may prescribe a controlled substance or,
specifically, buprenorphine and buprenorphine-naloxone. Although qualifying nurse
practitioners and physician assistants will now be allowed under federal law to receive a DATA
2000 waiver and prescribe buprenorphine, some states have explicit restrictions in place on
prescribing buprenorphine by non-physicians. These restrictions may be artifacts of states
Effects of Prior Authorization on
Medication Use and Costs
Research is limited regarding the impact of prior
authorization (PA) on medications for alcohol
and opioid use disorders. However, studies
have examined the effect of PA on medications
to treat other chronic conditions. Research on
the effect of PA on antipsychotics to treat
schizophrenia or bipolar disorder shows that PA
policies lead to higher rates of treatment
discontinuation and hospitalization and may
present barriers to access (Abouzaid et al.,
2010; Brown, Barrett, Caffery, Hourihan, &
Ireys, 2013; Lu et al., 2011; Zhang, Adams,
Ross-Degnan, Zhang, & Soumerai, 2009).
Formulary restrictions on atypical antipsychotics
have also been associated with higher total
medical expenditures and higher social costs
among Medicaid patients with schizophrenia
and bipolar disorder; these higher costs may
offset any savings in pharmacy costs (Seabury
et al., 2014).
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
49
attempting to ensure that their providers did not run afoul of the previous federal buprenorphine
restrictions. Failure to modify state licensure laws, however, to accommodate the prescribing
permissions found in current federal law may impede the increased access to buprenorphine and
buprenorphine-naloxone that the federal amendments sought to address. States face persistent
problems of insufficient buprenorphine prescriber capacity. Adjusting legacy statutes and
regulations to reflect the amended federal law could help alleviate that.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
50
V. Conclusion
A large body of research increasingly emphasizes that medications used in MAT are effective
for the treatment of alcohol and opioid use disorders and that naloxone is effective for
addressing acute opioid overdose. Studies also have shown that these medications are cost
effective and typically can pay for themselves. Given the scope of the opioid epidemic as well
as the toll that alcohol use disorders take on the population of the United States, coverage and
reimbursement policies that facilitate access to MAT are believed by many experts to be critical
elements of assisting persons with SUDs. As Medicaid programs continue to assess the needs
of individuals with alcohol and opioid use disorders in their states, this report can be a resource
guide for developing beneficial medication coverage and financing policies. Ongoing
assessment should include several different factors discussed below.
A. Coverage of Medications Used for MAT
Currently, the Medicaid programs in all states and the District of Columbia cover some form of
naloxone, oral and extended-release injectable naltrexone, buprenorphine, and buprenorphine-
naloxone, and disulfiram is covered in nearly all states.
20
In contrast, the Medicaid programs in
9 states still do not reimburse methadone, 11 do not reimburse acamprosate, 14 do not reimburse
implantable buprenorphine, and 18 do not reimburse extended-release injectable buprenorphine.
Careful examination of current coverage for medications, including methadone, is a critical step
toward combatting the opioid epidemic in the United States.
B. Benefit Design
Many Medicaid programs use benefit design requirements, such as prior authorization, to contain
expenditures and encourage the proper use of medications for the treatment of alcohol and opioid
disorders. These requirements may reduce patients’ access to treatment and result in poorer
health and economic treatment outcomes. Research on the use of prior authorization with
psychiatric medications has revealed that prior authorization may reduce medication
expenditures but also may have the unintended consequence of reducing use of the medication
and access to treatment.
Reconsideration of whether to require prior authorization or other benefit limitations is another
step states may wish to take toward addressing treatment access. Most benefit design restrictions
currently used by states are for medications to treat opioid use disorders. For example, prior
authorization is required for reimbursement of some form of buprenorphine and buprenorphine-
naloxone in 40 and 31 of the 51 Medicaid programs, respectively, and extended-release
injectable naltrexone requires prior authorization in 19 Medicaid programs. One state imposes a
20
We exclude Puerto Rico and the U.S. Virgin Islands from these numbers because of uncertainty about the status of
most of the drugs.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
51
blanket prior authorization requirement on all opioid use disorder treatments. However, state
Medicaid programs, health plans, and states acting via statute or regulation are increasingly
taking the step of removing prior authorization for MAT, particularly for treatment of opioid use
disorders, to facilitate access to urgently needed treatment.
One area in which reconsideration of prior authorization and other benefit limits may be most
important concerns extended-release injectable naltrexone. Because this medication remains on-
patent and there is no available generic, it is more expensive than most other MAT medications.
There is considerable evidence, however, that extended-release injectable naltrexone can be
effective and that it offers opportunities for treatment in settings such as the criminal justice
system or for populations such as the homeless, where access to or use of other MAT
medications may be more problematic. Because both methadone and the buprenorphine drugs
are so heavily regulated, oral naltrexone and extended-release injectable naltrexone offer a path
to treatment when access to either methadone or buprenorphine is limited. For this reason, close
examination of existing benefit limitations for extended-release injectable naltrexone is
important if states wish to ensure access to treatment.
C. Other Considerations
The recent amendments to federal statutes and regulations regarding prescribing of
buprenorphine were designed to open prescribing to include nurse practitioners and physician
assistants and to increase the number of patients a specially qualified prescriber may serve at one
time. Some state licensing laws, however, restrict scope of practice to preclude prescribing by
nurse practitioners and physician assistants, whereas other states have adopted regulations related
to buprenorphine prescribing that specifically refer only to physicians as buprenorphine
prescribers. To the extent that state laws or regulations inhibit prescribing of buprenorphine by
nurse practitioners or physician assistants, the intent of the federal changes will be unrealized.
Although states have a legitimate interest in regulating the scope of practice of providers in their
jurisdiction to keep their citizens safe and healthy and to regulate buprenorphine prescribing,
with the change in federal law, some states may want to re-examine which providers should be
permitted to prescribe MAT. These states with legacy scope of practice or buprenorphine
restrictions may consider amendments to state statutes and regulations to reflect current federal
law.
D. Lessons Learned from Innovative Approaches to Financing MAT.
The three innovative approaches to MAT from Massachusetts, Missouri, and Washington that
are highlighted in this report are just some of many underway in the United States. These three
represent several approaches and present a variety of opportunities and challenges that may be
useful to consider as other states assess their own financing of MAT. Importantly, all three of
the case study examples rely on multiple funding sources to support their innovative provision of
MAT, including Medicaid, to finance SUD services and medications. It is clear from these case
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
52
studies that reliance on Medicaid alone was insufficient to get these innovations under way and,
in some cases, will be insufficient to sustain them.
The Massachusetts model was built with a healthy boost of financing from the state in addition to
Medicaid reimbursement. Both funding sources were necessary to get the model going and to
facilitate sustainability. After initial development, however, at least the CHCs that are FQHCs
have managed to rely on Medicaid to cover salaries, administrative costs, and fringe benefits for
providers. The FQHCs, however, use a Medicaid prospective payment system that allows
greater flexibility in funding of services. Facilities without that advantage will have greater
difficulty in sustaining a Massachusetts-like model, suggesting that increased opportunities for
alternative payment approaches are needed to expand use of the Nurse Care Manager model and
that other states wishing to adopt this model also may need to find additional sources of money
to pay for it.
The state of Missouri also has relied on additional funding streams to supplement Medicaid
payments. Over the many years in which Missouri state government has worked to build and
sustain a statewide network of MAT providers, however, it learned that careful planning regarding
reimbursement rates was critical. Planning in advance for equitable reimbursement requires
setting rates that cover many things, including medications, the act of administration, laboratory
services, other MAT-related activities, and overhead to ensure that providers are fairly
compensated for the provision of MAT.
The Washington state Flex Care telemedicine pilot had the advantage of being able to use grant
funding to purchase its telehealth equipment and to fund other facets of the pilot. The pilot clinic
also is moving toward accepting insurance to assist in sustaining the pilot once grant funding
ends. Despite this, as a small rural clinic providing services to a large area, the pilot site still
struggles with finding staff and expects continued difficulty paying the salaries of the multiple
staff needed at both ends of the telemedicine encounter. This promising project, which now
provides MAT to more than 200 individuals who could not access treatment in the past, needs
the resources to sustain it. Other rural clinics and providers who may not have a grant to fund
telehealth equipment may not even be able to begin such a project.
Viable Medicaid reimbursement is a critical component of success for providers of MAT. As
Medicaid programs consider these and other innovative coverage and financing approaches, they
also must put in place viable coverage and benefit design structures that effectively reduce costs
but still improve access to necessary treatment and the quality of life for individuals with an
opioid or alcohol use disorder.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
53
VI. References
Abouzaid, S., Jutkowitz, E., Foley, K. A., Pizzi, L. T., Kim, E., & Bates, J. (2010). Economic
impact of prior authorization policies for atypical antipsychotics in the treatment of
schizophrenia. Population Health Management, 13(5), 247–254.
Accurso, A. J., & Rastegar, D. A. (2016). The effect of a payer-mandated decrease in
buprenorphine dose on aberrant drug tests and treatment retention among patients with
opioid dependence. Journal of Substance Abuse Treatment, 61, 74–79.
Addiction Treatment Forum. (2014, June 2). Project Lazarus brings opioid treatment program to
Wilkes County along with naloxone kits. Retrieved from
http://atforum.com/2014/06/project-lazarus-brings-opioid-treatment-program-to-wilkes-
county/
Alanis-Hirsch, K., Cross, R., Ford, J. H., Johnson, K., Chalk, M., Schmidt, L., & McCarty, D.
(2016). Extended-release naltrexone: A qualitative analysis of barriers to routine use.
Journal of Substance Abuse Treatment, 62, 68–73.
Agency for Healthcare Research and Quality. (2016). Medication-assisted treatment models of
care for opioid use disorder in primary care settings (Technical Brief No. 28; AHRQ
Publication No. 16(17)-EHC039-EF). Rockville, MD: Agency for Healthcare Research
and Quality. Retrieved from
https://effectivehealthcare.ahrq.gov/ehc/products/636/2350/opioid-use-disorder-report-
161123.pdf
Alford, D. P., LaBelle, C. T., Kretsch, N., Bergeron, A., Winter, M., Botticelli, M., & Samet, J.
F. (2011). Collaborative care of opioid-addicted patients in primary care using
buprenorphine: Five-year experience. Archives of Internal Medicine, 171(5), 425–431.
Amass, L., Pukeleviciene, V., Subata, E., Almeida, A. R., Pieri, M. C., D'Egidio, P, . . . Strang, J.
(2012). A prospective, randomized, multicenter acceptability and safety study of direct
buprenorphine/naloxone induction in heroin‐dependent individuals. Addiction, 107(1),
142–151.
American Association of Nurse Practitioners. (2017). 2017 nurse practitioner state practice
environment. From State Nurse State Practice Acts and Administration Rules, 2017.
Retrieved from https://www.aanp.org/images/documents/state-leg-
reg/stateregulatorymap.pdf
American Society of Addiction Medicine. (2013). Advancing access to addiction medications:
Implications for opioid addiction treatment. Retrieved from
https://www.asam.org/docs/default-source/advocacy/aaam_implications-for-opioid-
addiction-treatment_final
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
54
American Society of Addiction Medicine. (2015). The ASAM National Practice Guideline for the
Use of Medications in the Treatment of Addiction Involving Opioid Use. Retrieved from
https://www.asam.org/quality-practice/guidelines-and-consensus-documents/npg
Bachrach, D., Anthony, S., & Detty, A. (2014). State strategies for integrating physical and
behavioural health services in a changing medicaid environment. Mannatt, Phelps &
Phillips LLP, prepared for The Commonwealth Fund. Retrieved from
http://www.commonwealthfund.org/~/media/files/publications/fund-
report/2014/aug/1767_bachrach_state_strategies_integrating_phys_behavioral_hlt_827.p
df
Baser, O., Chalk, M., Fiellin, D. A., & Gastfriend, D. R. (2011). Cost and utilization outcomes of
opioid-dependence treatments. American Journal of Managed Care, 17(8), S235–S248.
Behavioral Health Integration Task Force. (2013). Report to the Legislature and the Health
Policy Commission. Retrieved from http://www.mass.gov/anf/docs/hpc/quipp/behavioral-
health-integration-task-force-final-report-and-recommendations-july-2013.pdf
Bouchery, E. E., Harwood, H. J., Sacks, J. J., Simon, C. J., & Brewer, R. D. (2011). Economic
costs of excessive alcohol consumption in the US, 2006. American Journal of Preventive
Medicine, 41(5), 516–524.
Brewer, C., Meyers, R. J., & Johnsen, J. (2000). Does disulfiram help to prevent relapse in
alcohol abuse? CNS Drugs, 14(5), 329–341.
Brolin, M., Quinn, A., Sirkin, J. T., Horgan, C. M., Parks, J., Easterday, J., & Levit, K. (2012).
Financing of behavioral health services within federally qualified health centers.
Retrieved from the Substance Abuse and Mental Health Services Administration website:
http://www.integration.samhsa.gov/Financing_BH_Services_at_FQHCs_Final_7_23-
12.pdf
Bronstein, A. C., Spyker, D. A., Cantilena, Jr., L. R., Green, J. L., Rumack, B. H., & Giffin, S. L.
(2009). 2008 Annual report of the American Association of Poison Control Centers’
National Poison Data System (NPDS): 26th annual report. Clinical Toxicology, 47(10),
911–1084.
Brooks, A. C., Comer, S. D., Sullivan, M. A., Bisaga, A., Carpenter, K. M., Raby, W. M., . . .
Nunes, E. V. (2010). Long-acting injectable versus oral naltrexone maintenance
therapy with psychosocial intervention for heroin dependence: a quasi-experiment.
Journal of Clinical Psychiatry, 71(10), 1371–1378.
Brown, J. D., Barrett, A., Caffery, E., Hourihan, K., & Ireys, H. T. (2013). Medication
continuity among Medicaid beneficiaries with schizophrenia and bipolar disorder.
Psychiatric Services, 64(9), 878–885.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
55
Bryson, W. C., McConnell, J., Korthuis, P. T., & McCarty D. (2011). Extended-release
naltrexone for alcohol dependence: Persistence and healthcare costs and utilization.
American Journal of Managed Care, 17(8), S222–S234.
Burris, S., Norland, J., & Edlin, B. R. (2001). Legal aspects of providing naloxone to heroin
users in the United States. International Journal of Drug Policy, 12, 237–248.
Bouza, C., Angeles, M., Ana, M., & María, A. J. (2004). Efficacy and safety of naltrexone and
acamprosate in the treatment of alcohol dependence: A systematic review. Addiction, 99,
811–828.
California Legislative Information. (n.d.). AB-1535 Pharmacists: Naloxone hydrochloride.
Retrieved from
http://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201320140AB1535
Center for Consumer Information and Insurance Oversight. (2013). The Mental Health Parity
and Addiction Equity Act fact sheet. Retrieved from
http://cciio.cms.gov/programs/protections/mhpaea/mhpaea_factsheet.html
Centers for Disease Control and Prevention. (2013). Alcohol and public health: Alcohol-Related
Disease Impact (ARDI). Average for United States 2006–2010 alcohol-attributable
deaths due to excessive alcohol use. Retrieved from
https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM&P=f6d7eda7-036e-
4553-9968-9b17ffad620e&R=d7a9b303-48e9-4440-bf47-070a4827e1fd&M=8E1C5233-
5640-4EE8-9247-1ECA7DA325B9&F=&D=
Centers for Medicare & Medicaid Services. (n.d.). 2016 Medicaid state drug utilization data.
[Web page]. Retrieved from https://www.medicaid.gov/medicaid/prescription-
drugs/state-drug-utilization-data/index.html
Centers for Medicare & Medicaid Services. (2014). Medication assisted treatment for substance
use disorders (CMCS informational bulletin). Retrieved from
https://www.medicaid.gov/Federal-Policy-Guidance/downloads/CIB-07-11-2014.pdf
Centers for Medicare & Medicaid Services. (2015). Coverage of behavioral health services for
youth with substance use disorders (CMCS informational bulletin). Retrieved from
https://www.medicaid.gov/Federal-Policy-Guidance/Downloads/CIB-01-26-2015.pdf
Centers for Medicare & Medicaid Services. (2017a). Reducing substance use disorders.
Retrieved from https://www.medicaid.gov/state-resource-center/innovation-accelerator-
program/reducing-substance-use-disorders/reducing-substance-use-disorders.html
Centers for Medicare & Medicaid Services. (2017b). State flexibility to facilitate timely access to
drug therapy by expanding the scope of pharmacy practice using collaborative practice

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
56
agreements, standing orders or other predetermined protocols (CMCS informational
bulletin). Retrieved from https://www.medicaid.gov/federal-policy-
guidance/downloads/cib011717.pdf
Centers for Medicare & Medicaid Services. (2018). Medicaid Drug Utilization Review State
Comparison/Summary Report FFY 2017: Annual Report Prescription Drug Fee-For-
Service Programs. Retrieved from https://www.medicaid.gov/.../2017-dur-summary-
report.pdf
Chamorro, A. J., Marcos, M., Mirón-Canelo, J. A., Pastor, I., González-Sarmiento, R., & Laso,
F. J. (2012). Association of μ-opioid receptor (OPRM1) gene polymorphism with
response to naltrexone in alcohol dependence: A systematic review and meta-analysis.
Addiction Biology, 17, 505–512.
Clapp, P. (2012). Current progress in pharmacologic treatment strategies for alcohol dependence.
Expert Reviews in Clinical Pharmacology, 5(4), 427–435.
Clark, R. E., Baxter, J. D., Barton, B. A., Aweh, G., O’Connell, E., & Fisher, W. H. (2014). The
impact of prior authorization on buprenorphine dose, relapse rates, and cost for
Massachusetts Medicaid beneficiaries with opioid dependence. Health Services Research,
49(6), 1964–1979.
Clark, R. E., & Baxter, J. D. (2013). Responses of state Medicaid programs to buprenorphine
diversion: Doing more harm than good? JAMA Internal Medicine, 173(17), 1571–1572.
Clark, R. E., Samnaliev, M., Baxter, J. D., & Leung, G. Y. (2011). The evidence doesn’t justify
steps by state Medicaid programs to restrict opioid addiction treatment with
buprenorphine. Health Affairs (Millwood), 30(8), 1425–1433.
Commonwealth of Pennsylvania. (2015, October 29). Governor Wolf announces naloxone
standing order to combat heroin epidemic (+ FAQs). Retrieved from
https://www.governor.pa.gov/naloxone-standing-order/
Comprehensive Addiction and Recovery Act of 2016, 21 U.S.C. § 303 (2016).
Connock, M., Juarez-Garcia, A., Jowett, S., Frew, E., Liu, Z., Taylor, R. J., . . . Taylor, R. S.
(2007). Methadone and buprenorphine for the management of opioid dependence: A
systematic review and economic evaluation. Health Technology Assessment, 11(9), 1–
171, iii–iv.
Controlled Substances Act, 21 U.S.C. § 812 (1970).
CoverMyMeds. (2017). ePA National Adoption Scorecard. Columbus, OH: CoverMyMeds.
Retrieved from https://epascorecard.covermymeds.com/

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
57
Cunningham, P. J., & Malley, A. S. O. (2009). Do reimbursement delays discourage Medicaid
participation by physicians? Health Affairs (Millwood), 28(1), w17–w28.
Davis, C. S., Ruiz, S., Glynn, P., Picariello, G., & Walley, A. Y. (2014). Expanded access to
naloxone among firefighters, police officers, and emergency medical technicians in
Massachusetts. American Journal of Public Health, 104(8), e7–e9.
Department of Health and Human Services. (2016). Final rules under the Medicaid and
Children's Health Insurance Programs; Mental Health Parity and Addiction Equity Act of
2008; the Application of Mental Health Parity Requirements to Coverage Offered by
Medicaid Managed Care Organizations, the Children’s Health Insurance Program
(CHIP), and Alternative Benefit Plans; Final rule. 81 Fed. Reg. 61, 18390.
Department of the Treasury, Department of Labor, Department of Health and Human Services.
(2013). Final rules under the Paul Wellstone and Pete Domenici Mental Health Parity and
Addiction Equity Act of 2008; Final rule. 78 Fed. Reg. 219, 68239.
Department of Vermont Health Access. (2017). Pharmacy benefit management program.
Retrieved from http://dvha.vermont.gov/for-providers/vermont-pdl-effective-01-01-17-
dec-minutes-2016.final.v3.pdf
Devlin, R. J., & Henry, J. A. (2008). Clinical review: Major consequences of illicit drug
consumption. Critical Care, 12(1), 202.
Donoghue, K., Elzerbi, C., Saunders, R., Whittington, C., Pilling, S., & Drummond, C. (2015).
The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence,
Europe versus the rest of the world: a meta-analysis. Addiction, 110(6), 920–930.
Drug Addiction Treatment Act of 2000, 21 U.S.C. (2000); as amended by the Office of National
Drug Control Policy Reauthorization Act of 2006, 21 U.S.C. § 1102 (2006).
Drug Policy Alignment. (n.d.). Expanding access to naloxone: Reducing fatal overdose, saving
lives. Retrieved from
http://www.drugpolicy.org/sites/default/files/DPA_Naloxone_Issue%20Brief_0.pdf
Dunlop, A. J., Brown, A. L., Oldmeadow, C., Harris, A., Gill, A., Sadler, C., . . . Lintzeris, N.
(2017). Effectiveness and cost-effectiveness of unsupervised buprenorphine-naloxone for
the treatment of heroin dependence in a randomized waitlist controlled trial. Drug and
Alcohol Dependence, 174, 181–191.
Faggiano, F., Vigna-Taglianti, F., Versino, E., & Lemma, P. (2003). Methadone maintenance at
different dosages for opioid dependence. Cochrane Database System Reviews, 2003(1),
CD002208.
Federal Opioid Treatment Standards, 42 CFR § 8.12. (2002).

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
58
Florence, C. S., Zhou, C., Luo, F., & Xu, L. (2016). The economic burden of prescription opioid
overdose, abuse, and dependence in the United States, 2013. Medical Care, 54(10):901–
906.
Food and Drug Administration. (2015, November 18). FDA moves quickly to approve easy-to-
use nasal spray to treat opioid overdose [FDA news release]. Retrieved from
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm473505.htm
Food and Drug Administration. (2016a). FDA approves first buprenorphine implant for
treatment of opioid dependence. Retrieved from
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm503719
Food and Drug Administration. (2016b). FDA’s naloxone app prize competition celebrates
innovation in search of technological solutions to the opioid epidemic. Retrieved from
https://blogs.fda.gov/fdavoice/index.php/2016/12/fdas-naloxone-app-prize-competition-
celebrates-innovation-in-search-of-technological-solutions-to-the-opioid-epidemic/
Food and Drug Administration. (2017a) Understanding unapproved use of approved drugs “off
label.” Retrieved from https://www.fda.gov/forpatients/other/offlabel/default.htm
Food and Drug Administration. (2017b). Frequently Asked Questions about CDER. Retrieved
from
https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/C
DER/FAQsaboutCDER/#top
Food and Drug Administration. (2017c). Generic drugs. Retrieved from
https://www.fda.gov/Drugs/resourcesforyou/consumers/buyingusingmedicinesafely/unde
rstandinggenericdrugs/default.htm
Food and Drug Administration. (2017d). Facts about generic drugs. Retrieved from
https://www.fda.gov/drugs/resourcesforyou/consumers/buyingusingmedicinesafely/under
standinggenericdrugs/ucm167991.htm
Food and Drug Administration. (2017e). FDA approves first once-monthly buprenorphine
injection, a medication-assisted treatment option for opioid use disorder. Retrieved from
https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm587312.htm
Formulary Watch. (2013, February 25). FDA approves first generic Suboxone for maintenance
treatment of opioid drug dependence. Retrieved from
http://formularyjournal.modernmedicine.com/formulary-journal/news/formulary/fda-
approvals/fda-approves-first-generic-suboxone-maintenance-treat?page=full
Fullerton, C. A., Kim, M., Thomas, C. P., Lyman, D. R., Montejano, L. B., Dougherty, R. H., . . .
Delphin-Rittmon, M. E. (2014). Medication-assisted treatment with methadone:
Assessing the evidence. Psychiatric Services, 65(2), 146–157.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
59
Garbutt, J. C. (2009). The state of pharmacotherapy for the treatment of alcohol dependence.
Journal of Substance Abuse Treatment, 36(1), S15–S25.
Garbutt, J. C., Kranzler, H. R., Malley, S. S., Gastfriend, D. R., Pettinati, H. M., Silverman B. L.,
. . . Vivitrex Study Group. (2005). Efficacy and tolerability of long-acting injectable
naltrexone. Journal of the American Medical Association, 293(13), 1617–1625.
Gowing, L., Farrell, M. F., Bornemann, R., Sullivan, L. E., & Ali, R. (2011). Oral substitution
treatment of injecting opioid users for prevention of HIV infection. Cochrane Database
System Reviews, 2011(8), CD004145.
Gupta, R., Shah, N.D., & Ross, J.S. (2016). The rising price of naloxone—Risks to efforts to
stem overdose deaths. New England Journal of Medicine, 375(23), 2213-2215.
Harm Reduction Coalition. (n.d.). Naloxone program case studies. Retrieved from
http://harmreduction.org/issues/overdose-prevention/tools-best-practices/naloxone-
program-case-studies/
Harris, A. H. S., Bowe, T., Del Re, A. C., Finlay, A. K., Oliva, E., Myrick, H. L., & Rubinsky,
A. D. (2015). Extended release naltrexone for alcohol use disorders: Quasi-experimental
effects on mortality and subsequent detoxification episodes. Alcoholism: Clinical and
Experimental Research, 39(1), 79–83.
Hartung, D. M., McCarty, D., Fu, R., Wiest, K., Chalk, M., & Gastfriend, D. R. (2014).
Extended-release naltrexone for alcohol and opioid dependence: A meta-analysis of
healthcare utilization studies. Journal of Substance Abuse Treatment, 47(2), 113–121.
Hawk, K. F, Vaca, F. E., & D’Onofrio, G. (2015). Reducing fatal opioid overdose: Prevention,
treatment and harm reduction strategies. Yale Journal of Biology and Medicine, 88, 235–
245.
Helstrom, A. W., Blow, F. C., Slaymaker, V., Kranzler, H. R., Leong, S., & Oslin, D. (2016).
Reductions in alcohol craving following naltrexone treatment for heavy drinking. Alcohol
and Alcoholism, 51(5), 562–566.
Jackson, H., Mandell, K., Johnson, K., Chatterjee, D., & Vanness, D. J. (2015). Cost
effectiveness of injectable extended release naltrexone compared to methadone
maintenance and buprenorphine maintenance treatment for opioid dependence. Substance
Abuse, 36, 226–231.
Johansson, B. A., Berglund, M., & Lindgren, A. (2006). Efficacy of maintenance treatment with
naltrexone for opioid dependence: A meta-analytical review. Addiction, 101, 491–503.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
60
Johnson, R. E., Chutuape, M. A., Strain, E. C., Walsh, S. L., Stitzer, M. L., & Bigelow, G. E.
(2000). A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid
dependence. New England Journal of Medicine, 343(18), 1290–1297.
Jonas, D. E., Amick, H. R., Feltner, C., Bobashev, G., Thomas, K., Wines, R., . . . Garbutt, J. C.
(2014). Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A
systematic review and meta-analysis. JAMA, 311(18), 1889–1900.
Jorgensen, C. H., Pedersen, B., & Tonnesen, H. (2011). The efficacy of disulfiram for the
treatment of alcohol use disorder. Alcoholism: Clinical and Experimental Research,
35(10), 1749–1758.
Justia. (n.d.). 2015 Oklahoma Statutes Title 63. Public Health and Safety §63-2-312.2. Sale or
dispensation of naloxone. Retrieved from
http://law.justia.com/codes/oklahoma/2015/title-63/section-63-2-312.2/
Justia. (2016). 2010 New York Code PBH-Public Health Article 33-Controlled substances.
Retrieved from http://law.justia.com/codes/new-york/2010/pbh/article-33
Kaiser Family Foundation. (2017). Medicaid moving ahead in uncertain times: Results from a
50-state Medicaid budget survey for state fiscal years 2017 and 2018. Retrieved from
http://files.kff.org/attachment/Report-Results-from-a-50-State-Medicaid-Budget-Survey-
for-State-Fiscal-Years-2017-and-2018
Kaiser Family Foundation. (2018). State health facts: States reporting Medicaid coverage of
medication assisted treatment (MAT) drugs. Retrieved from
https://www.kff.org/medicaid/state-indicator/states-reporting-medicaid-coverage-of-
medication-assisted-treatment-mat-
drugs/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort
%22:%22asc%22%7D
Kelty, E., Hayes, L., O’Neil, G., Kyle, S., Jeffrey, G. P., O'Neil, G., . . . Hulse, G. (2014).
Changes in hospital and out-patient events and costs following implant naltrexone
treatment for problematic alcohol use. Journal of Psychopharmacology, 28(8), 745–750.
Kinlock, T. W., Gordon, M. S., Schwartz, R. P., O’Grady, K., Fitzgerald, T. T., & Wilson, M.
(2007). A randomized clinical trial of methadone maintenance for prisoners: Results at 1-
month post-release. Drug and Alcohol Dependence, 91(2–3), 220–227.
Laaksonen, E., Koski-Jannes, A., Salaspuro, M., Ahtinen, H., & Alho, H. (2008). A randomized,
multicentre, open-label, comparative trial of disulfiram, naltrexone and acamprosate in
the treatment of alcohol dependence. Alcohol and Alcoholism, 43, 53–61.
LaBelle, C. T., Han, S. C., Bergeron, A., & Samet, J. H. (2016). Office-based opioid treatment
with buprenorphine (OBOT-B): Statewide implementation of the Massachusetts

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
61
collaborative care model in community health centers. Journal of Substance Abuse
Treatment, 60, 6–13.
Leavitt, S. B. (2002). Evidence for the efficacy of naltrexone in the treatment of alcohol
dependence (alcoholism). Addiction Treatment Forum. Retrieved from
http://atforum.com/pdf/NaltrexoneWhitePaper.pdf
Lee, J., Kresina, T. F., Campopiano, M., Lubran, R., & Clark, H. W. (2015). Use of
pharmacotherapies in the treatment of alcohol use disorders and opioid dependence in
primary care. BioMed Research International, 2015, 137020.
Lee, J. D., Friedmann, P. D., Kinlock, T. W., Nunes, E. V., Boney, T. Y., Hoskinson, R. A., . . .
O’Brien, C. P. (2016). Extended-release naltrexone to prevent opioid relapse in criminal
justice offenders. New England Journal of Medicine, 374(13), 1232–1242.
Legal Systems, LLC. (2016). Prescription Drug Abuse Policy System. Retrieved from
http://www.pdaps.org/
Lobmaier, P. P., Kunøe, N., Gossop, M., & Waal, H. (2011). Naltrexone depot formulations for
opioid and alcohol dependence: A systematic review. CNS Neuroscience and
Therapeutics, 17, 629–636.
Lu, C. Y., Adams, A. S., Ross-Degnan, D., Zhang, F., Zhang, Y., Salzman, C., & Soumerai, S.
B. (2011). Association between prior authorization for psychiatric medications and use of
health services among Medicaid patients with bipolar disorder. Psychiatric Services,
62(2), 186–193.
Maisel, N. C., Blodgett, J. C., Wilbourne, P. L., Humphreys, K., & Finney, J. W. (2013). Meta-
analysis of naltrexone and acamprosate for treating alcohol use disorders: When are these
medications most helpful? Addiction, 108(2), 275–293.
Mann, K. (2004). Pharmacotherapy of alcohol dependence: A review of the clinical data. CNS
Drugs, 18, 485–504.
Mann, K., Lehert, P., & Morgan, M. Y. (2004). The efficacy of acamprosate in the maintenance
of abstinence in alcohol-dependent individuals: Results of a meta-analysis. Alcoholism:
Clinical and Experimental Research, 28, 51–63.
Mannelli, P., Peindl, K., Masand, P. S., & Patkar, A. A. (2007). Long-acting injectable
naltrexone for the treatment of alcohol dependence. Expert Reviews in Neurotherapy,
7(10), 1265–1277.
Mark, T. L., Montejano, L. B., Kranzler, H. R., Chalk, M., & Gastfriend, D. R. (2010).
Comparison of healthcare utilization among patients treated with alcoholism medications.
American Journal of Managed Care, 16(12), 879–888.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
62
Mason, B. J., & Lehert, P. (2012). Acamprosate for alcohol dependence: A sex-specific meta-
analysis based on individual patient data. Alcohol Clinical Experimental Research, 36(3),
497–508.
Massachusetts Department of Public Health. (2016). 105 CMR: Department of Public Health
(MA Reg. #1305). Retrieved from
http://www.mass.gov/eohhs/docs/dph/regs/105cmr164.pdf
Mattick, R. P., Breen, C., Kimber, J., & Davoli, M. (2014). Buprenorphine maintenance versus
placebo or methadone maintenance for opioid dependence. Cochrane Database System
Reviews, 2, CD002207.
Mauch, D., Kautz, C., & Smith. S. (2008). Reimbursement of mental health services in primary
care settings (HHS Publication No. SMA-08-4324). Retrieved from Substance Abuse and
Mental Health Services Administration website:
http://www.integration.samhsa.gov/Reimbursement_of_Mental_Health_Services_in_Pri
mary_Care_Settings.pdf
McLellan, A. T., Lewis, D. C., O'Brien, C. P., & Kleber, H. D. (2000). Drug dependence, a
chronic medical illness: Implications for treatment, insurance, and outcomes evaluation.
JAMA, 284(13), 1689–1695.
Medication Assisted Treatment for Opioid Use Disorders, 42 Fed. Reg. § 8 (2016).
Mueller, S. R. Walley, A. Y., Calcaterra, S. L., Glanz, J. M., & Binswanger, I. A. (2015). A
review of opioid overdose prevention and naloxone prescribing: Implications for
translating community programming into clinical practice. Substance Abuse, 36(2), 240-
253.
Muller, A. (2016). Naloxone: The science and laws behind the antidote. ABA Heath eSource,
13(1).
Murphy, S. M., & Neven, D. (2014). Cost-effective: Emergency department care coordination
with a regional hospital information system. The Journal of Emergency Medicine, 47(2),
223–231.
National Conference of State Legislatures. (2015). Mental health benefits: State laws mandating
or regulating. Retrieved from http://www.ncsl.org/research/health/mental-health-
benefits-state-mandates.aspx#3
National Conference of State Legislatures. (2017). Drug overdose immunity and good samaritan
laws. Retrieved from http://www.ncsl.org/research/civil-and-criminal-justice/drug-
overdose-immunity-good-samaritan-laws.aspx
National Insitute on Alcohol Abuse and Alcoholism. (2008). Excerpt from “Helping patients
who drink too much: A clinician’s guide” (NIH Publication 07–3769). Retrieved from

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
63
https://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/PrescribingMed
s.pdf
National Institute on Drug Abuse. (2012a). Medical consequences of drug abuse. Retrieved from
http://www.drugabuse.gov/related-topics/medical-consequences-drug-abuse
National Institute on Drug Abuse. (2012b). How effective is drug treatment? In Principles of
drug addiction treatment: a research-based guide (3rd ed.; NIH Publication No. 12-
4180). Bethesda, MD: National Institutes of Health. Retrieved from
https://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-
based-guide-third-edition/frequently-asked-questions/how-effective-drug-addiction-
treatment
National Institute on Drug Abuse. (2012c). Drug addiction is a complex illness. In Principles of
drug addiction treatment: A research-based guide (3rd ed.; NIH Publication No. 12-
4180). Bethesda, MD: National Institutes of Health. Retrieved from
https://www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-
based-guide-third-edition
National Institute on Drug Abuse. (2016a). The science of drug abuse and addiction: The basics.
Retrieved from https://www.drugabuse.gov/publications/media-guide/science-drug-
abuse-addiction-basics
National Institute on Drug Abuse. (2016b). Treatment approaches for drug addiction. Retrieved
from https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-
addiction
National Institute on Drug Abuse. (2018). Overdose death rates. Retrieved from
https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
National Library of Medicine. (2017). Buprenorphine HCl tablet. Retrieved from
http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=1bf8b35a-b769-465c-a2f8-
099868dfcd2f
New York State Department of Health. (n.d.). Opioid overdose prevention programs, Section
80.138 regulations. Retrieved from
https://www.health.ny.gov/diseases/aids/general/opioid_overdose_prevention/regulations.
htm
New York State Department of Health. (2016). NYS Medicaid FFS Program nalaxone coverage
through a non-patient specific (standing order) prescription. Retrieved from
https://newyork.fhsc.com/downloads/providers/NYRx_PDP_prescriber_notification_201
60311.pdf
NYU Langone Medical Center. (2016, March 30). Opioid relapse rates fall with long-term use of
medication for adults involved in criminal justice system [Press release]. Retrieved from
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
64
http://nyulangone.org/press-releases/opioid-relapse-rates-fall-with-long-term-use-of-
medication-for-adults-involved-in-criminal-justice-system
North Dakota Department of Human Services. (2016, June 27). Stop overdose: Naloxone access
expanded to ND pharmacies. Retrieved from https://www.nd.gov/dhs/info/news/2016/6-
27-stop-overdose-naloxone-available-at-participating-nd-pharmacies.pdf
O’Connor, P. G., & Fiellin, D. A. (2000). Pharmacologic treatment of heroin-dependent patients.
Annals of Internal Medicine, 133, 40–54.
Oregon Health and Science University. (2015, July). Best practices in naloxone treatment
programs for opioid overdose. Retrieved from https://www.ohsu.edu/xd/research/centers-
institutes/evidence-based-policy-
center/evidence/med/upload/MED_best_practices_naloxone_report_final.pdf
Oregon State Legislature. (2016). Section 4 in Oregon Laws 2016. Retrieved from
https://www.oregonlegislature.gov/bills_laws/lawsstatutes/2016orLaw0100.pdf
ParityTrack. (n.d.). Parity implementation national survey. Retrieved from
https://paritytrack.org/parity-reports/state-reports/
The Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008
(MHPAEA), 29 U.S.C. § 1185a (2008).
Pettinati, H. M., O’Brien, C. P., Rabinowitz, A. R., Wortman, S. P., Oslin, D. W., Kampman, K.
M., & Dackis, C. A. (2006). The status of naltrexone in the treatment of alcohol
dependence: Specific effects on heavy drinking. Journal of Clinical
Psychopharmacology, 26(6), 610–625.
Policy Surveillance Program. (2016). Naloxone overdose prevention laws. Retrieved from
http://lawatlas.org/query?dataset=laws-regulating-administration-of-naloxone
Rettig, R. A., & Yarmolinsky, A. (Eds.). (1995). Federal regulations of methadone treatment.
Institute of Medicine, Washington, DC: The National Academies Press.
Rösner, S., Hackl-Herrwerth, A., Leucht, S., Lehert, P., Vecchi, S., & Soyka, M. (2011).
Acamprosate for alcohol dependence. Library (London), 2, CD004332.
Sacks, J. J., Gonzales, K. R., Bouchery, E. E., Tomedi, L. E., & Brewer, R. D. (2010). National
and state costs of excessive alcohol consumption. American Journal of Preventive
Medicine, 49(5), e73–e79.
Schwartz, R. P., Gryczynski, J., O’Grady, K. E., Sharfstein, J. M., Warren, G., Olsen, Y., . . .
Jaffe, J. H. (2013). Opioid agonist treatments and heroin overdose deaths in Baltimore,
Maryland, 1995-2009. American Journal of Public Health, 103(5), 917–922.
Seabury, S. A., Goldman, D. P, Kalsekar I., Sheehan, J. J., Laubmeier, K., & Lakdawalla, D. N.
(2014). Formulary restrictions on atypical antipsychotics: Impact on costs for patients

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
65
with schizophrenia and bipolar disorder in Medicaid. American Journal of Managed
Care, 20(2), e52–e60.
Seth, P., Scholl, L., Rudd, R. A., & Bacon, S. (2018). Overdose deaths involving opioids,
cocaine, and psychostimulants — United States, 2015–2016. Morbidity and Mortality
Weekly Report, Centers for Disease Control and Prevention, 67(12), 349-358. Retrieved
from https://www.cdc.gov/mmwr/volumes/67/wr/mm6712a1.htm
Smith, V. K., Gifford, K., Ellis, E., Edwards, B., Rudowitz, R., Hinton, E., . . . Valentine, A.
(2016). Implementing coverage and payment initiatives: Results from a 50-state
Medicaid budget survey for state fiscal years 2016 and 2017. The Henry J. Kaiser Family
Foundation. Retrieved from http://kff.org/report-section/implementing-coverage-and-
payment-initiatives-benefits-and-pharmacy/
Sordo, L., Barrio, G., Bravo, M. J., Iciar Indave, B., Degenhardt, L., Wiessing, L., . . . Pastor-
Barriuso, R. (2017). Mortality risk during and after opioid substitution treatment:
Systematic review and meta-analysis of cohort studies. British Medical Journal, 7,
357:j1550.
Soyka, M., Zingg, C., Koller, G., & Kuefner, H. (2008). Retention rate and substance use in
methadone and buprenorphine maintenance therapy and predictors of outcome: Results
from a randomized study. International Journal of Neuropsychopharmacology, 11, 641–
653.
Specka, M., Heilmann, M., Lieb, B., & Scherbaum, N. (2014). Use of disulfiram for alcohol
relapse prevention in patients in opioid maintenance treatment. Clinical
Neuropharmacology, 37, 161–165.
State of Delaware. (2014). §3001D(a) in Chapter 384 formerly House Bill No. 388 as amended
by House Amendment No. 1. Retrieved from
http://delcode.delaware.gov/sessionlaws/ga147/chp384.shtml
State of South Dakota. (2015). Section 1 or Senate Bill No. 14. Retrieved from
http://www.sdlegislature.gov/docs/legsession/2015/Bills/SB14P.pdf
Stowkowski, L.A. (2016). APRN prescribing law: A state-by-state summary. Medscape.
Retrieved from http://www.medscape.com/viewarticle/440315
Substance Abuse and Mental Health Services Administration. (2009). Incorporating alcohol
pharmacotherapies into medical practice: Treatment Improvement Protocol (TIP) series
49. Retrieved from http://store.samhsa.gov/product/TIP-49-Incorporating-Alcohol-
PharmacotherapiesInto-Medical-Practice/SMA12-4380
Substance Abuse and Mental Health Services Administration. (2012). TIP 43: Medication-
assisted treatment for opioid addiction in opioid treatment programs. Retrieved from
https://store.samhsa.gov/shin/content//SMA12-4214/SMA12-4214.pdf
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
66
Substance Abuse and Mental Health Services Administration. (2014a). Prevention of substance
abuse and mental illness. Retrieved from http://www.samhsa.gov/prevention
Substance Abuse and Mental Health Services Administration. (2014b). Projections of National
Expenditures for Treatment of Mental and Substance Use Disorders, 2010-2020.
Retrieved from https://store.samhsa.gov/shin/content//SMA14-4883/SMA14-4883.pdf
Substance Abuse and Mental Health Services Administration. (2014c). Medicaid coverage and
financing of medications to treat alcohol and opioid use disorders. Retrieved from
http://store.samhsa.gov/shin/content/SMA14-4854/SMA14-4854.pdf
Substance Abuse and Mental Health Services Administration. (2015). Federal guidelines for
opioid treatment programs. Retrieved from http://store.samhsa.gov/shin/content//PEP15-
FEDGUIDEOTP/PEP15-FEDGUIDEOTP.pdf
Substance Abuse and Mental Health Services Administration. (2016a). Buprenorphine.
Retrieved from https://www.samhsa.gov/medication-assisted-
treatment/treatment/buprenorphine
Substance Abuse and Mental Health Services Administration. (2016b). SAMHSA opioid
overdose prevention toolkit. Retrieved from https://store.samhsa.gov/product/SAMHSA-
Opioid-Overdose-Prevention-Toolkit/SMA16-4742
Substance Abuse and Mental Health Services Administration. (2016c). Qualify for a physician
waiver. Retrieved from https://www.samhsa.gov/medication-assisted-
treatment/buprenorphine-waiver-management/qualify-for-physician-waiver
Substance Abuse and Mental Health Services Administration. Center for Behavioral Health
Statistics and Quality. (2017). Results from the 2016 National Survey on Drug Use and
Health: Detailed tables. Prevalence estimates, standard errors, P values, and sample
sizes. Retrieved from https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-
2016/NSDUH-DetTabs-2016.pdf
Suh, J. J., Pettinati, H. M., Kampman, K. M., & O’Brien, C. P. (2006). The status of disulfiram:
A half of a century later. Journal of Clinical Psychopharmacology, 26(3), 290–302.
Swift, R., Oslin, D. W., Alexander, M., & Forman, R. (2011). Adherence monitoring in
naltrexone pharmacotherapy trials: A systematic review. Journal of the Study of Alcohol
and Drugs, 72(6), 1012–1018.
Terkel, A., & Cherkis, J. (2015, August 25). Maine drug addiction clinic to close, blaming Gov.
Paul LePage’s policies. The Huffington Post. Retrieved from
http://www.huffingtonpost.com/entry/paul-lepage-methadone-
clinic_us_55dc8ab2e4b04ae4970478cc
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
67
Texas Health and Human Services, Texas Department of State Health Services. (2016). Program
news—Substance abuse treatment facilities. Retrieved from
http://www.dshs.texas.gov/facilities/substance-abuse/news.aspx
Thomas, C. P., Fullerton, C. A., Kim, M., Montejano, L., Lyman, D. R., Dougherty, R. H., . . .
Delphin-Rittmon, M. E. (2014). Medication-assisted treatment with buprenorphine:
Assessing the evidence. Psychiatric Services, 65(2), 158–170.
Tkacz, J., Volpicelli, J., Un, H., & Ruetsch, C. (2014). Relationship between buprenorphine
adherence and health service utilization and costs among opioid dependent patients.
Journal of Substance Abuse Treatment, 46(4), 456–462.
Urban Institute. (2017). Medicaid coverage of effective treatment for opioid use disorder.
Retrieved from
http://www.urban.org/sites/default/files/publication/90461/2001287_medicaid_coverage_
of_effective_treatment_for_opioid_use_disorder_1.pdf
U.S. Department of Justice, Drug Enforcement Administration, Diversion Control Division.
(n.d.) Title 21. Code of Federal Regulations, Part 1308 – Schedules of controlled
substances. Retrieved from https://www.deadiversion.usdoj.gov/21cfr/cfr/2108cfrt.htm
U.S. News and World Report. (2016). Coming to a high school near you: Drugs that reverse
heroin overdoses. Retrieved from https://www.usnews.com/news/articles/2016-01-
25/overdose-reversal-drug-naloxone-offered-free-to-high-schools
UNM School of Medicine. (2017). Project Echo: Opioid addiction treatment [Web page].
Retrieved from http://echo.unm.edu/nm-teleecho-clinics/opioid/
van Dorp, E., Yassen, A., & Dahan, A. (2007). Naloxone treatment in opioid addiction: The risks
and benefits. Expert Opinion in Drug Safety, 6(2), 125–132.
Vestal, C. (2017, April 21). Nurse licensing laws block treatment for opioid addiction. The Pew
Charitable Trusts, Stateline. Retrieved from http://www.pewtrusts.org/en/research-and-
analysis/blogs/stateline/2017/04/21/nurse-licensing-laws-block-treatment-for-opioid-
addiction?utm_campaign=2017-04-
21+Stateline+Daily&utm_medium=email&utm_source=Pew
Volpicelli, J. R., Rhines, K. C., Rhines, J. S., Volpicelli, L. A, Alterman, A. I., & O’Brien, C. P.
(1997). Naltrexone and alcohol dependence. Role of subject compliance. Archives of
General Psychiatry, 54(8), 737–742.
Weiss, R. D., Potter, J. S., Fiellin, D. A., Byrne, M., Connery, H. S., Dickinson, W., . . . Ling W.
(2011). Adjunctive counseling during brief and extended buprenorphine-naloxone
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
68
treatment for prescription opioid dependence: A 2-phase randomized controlled trial.
Archives of General Psychiatry, 68(12), 1238–1246.
White, A. G., Birnbaum, H. G., Mareva, M. N., Daher, M., Vallow, S., Schein, J., & Katz, N.
(2005). Direct costs of opioid abuse in an insured population in the United States. Journal
of Managed Care & Specialty Pharmacy, 11(6), 469–479.
Wickramatilake, S., Zur, J., Mulvaney-Day, N., Klimo, M.C.V., Selmi, E., & Harwood, H.
(2017). How states are tackling the opioid crisis. Public Health Reports, 132(2), 171–
179.
Woody, G. E., Bruce, D., Korthuis, P. T. Chhatre, S., Poole, S., Hillhouse, M., . . . Ling, W.
(2015). HIV risk reduction with buprenorphine-naloxone or methadone: Findings from a
randomized trial. Journal of Acquired Immune Deficiency Syndrome, 68(5), 554–561.
Zaric, G. S., Brandeau, M. L., & Barnett P. G. (2000). Methadone maintenance and HIV
prevention: a cost-effectiveness analysis. Management Science, 46(8), 1013–1031.
Zhang, Y., Adams, A. S., Ross-Degnan, D., Zhang, F., & Soumerai, S. (2009). Effects of prior
authorization on medication discontinuation among Medicaid beneficiaries with bipolar
disorder. Psychiatric Services, 60(4), 520–527.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
69
VII. Appendix A. Coverage of Medications for Alcohol and
Opioid Use Disorders by State
Table A-1. State Medicaid Documents Used to Identify Medication Coverage for Alcohol and
Opioid Use Disorders or to Reverse Opioid Overdose
State
Effective Date
Document Name
Link
Alabama
07/02/2018
Alabama PDL
Link
Accessed
08/10/2018
Alabama Drug Look-up Link
08/01/2017
Alabama Rehabilitative Option Fee Schedule
Link
Alaska
03/15/2015
Alaska PDL
Link
05/08/2013
Alaska Prior Authorization
Link
09/29/2017 Alaska Interim Prior Authorization Link
Accessed
08/10/2018
Alaska Medication Prior Authorization Webpage Link
10/03/2016
Alaska Medicaid Prior Authorization—Evzio
Link
03/19/2010
Alaska Medicaid Suboxone and Subutex (Buprenorphine)
Link
Accessed
07/31/2018
Kaiser Family Foundation States Reporting Medicaid Coverage of
MAT Drugs
Link
Arizona
Accessed
08/10/2018
Arizona Pharmacy Information
Link
07/01/2018
Arizona Acute/Long Term Care PDL
Link
07/01/2018
Arizona Buprenorphine Use in Pregnant and Nursing Women for
Medication Assisted Therapy
Link
07/01/2018
Arizona Behavioral Health Drug List
Link
11/2017
Arizona Health Care Cost Containment System Covered
Behavioral Health Services Guide
Link
Arkansas
08/10/2018
Arkansas PDL
Link
07/10/2018
Arkansas Prior Authorization
Link
Accessed
08/09/2018
Arkansas Drug Lookup Link
05/11/2017
Arkansas Office-Based Opioid Dependence Pharmacotherapy
Statement of Medical Necessity
Link
02/20/2017
Arkansas Medicaid Prior Authorization edits—
buprenorphine-containing agents
Link
California
Accessed
08/09/2018
California PDL (contract drug list) Link
08/01/2018
California Health and Wellness PDL
Link
Accessed
08/09/2018
California Online PDL Link
Amended
04/05/2018
California Special Terms and Conditions Section 1115 Waiver Link
04/2016
California Heroin Detoxification
Link
Colorado
07/01/2018
Colorado PDL
Link
07/01/2018
Colorado Prior Authorization and Quantity Limits
Link
06/01/2018
Colorado Access Child Health Plan+ Formulary
Link
07/01/2018
Colorado Uniform Service Coding Standards Manual
Link
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
70
State
Effective Date
Document Name
Link
Connecticut
07/01/2018
Connecticut PDL
Link
Accessed
07/16/2018
Connecticut Behavioral Health Partnership Services
Link
10/2018
Medicaid Drug Utilization Review State Comparison/Summary
Report FFY 2017 Annual Report Prescription Drug Fee-For-
Service Programs
Link
Delaware
07/03/2018
Delaware PDL
Link
Accessed
08/20/2018
Delaware Health Options Supplemental Formulary Link
12/23/2016
Delaware Medicaid and Medical Assistance Request for Prior
Authorization Vivitrol
Link
Accessed
07/12/2018
Delaware Procedure Codes
Link
District of
Columbia
07/12/2018
Washington DC PDL
Link
Accessed
07/28/2018
Washington DC Fee Schedule
Link
Accessed
08/07/2018
Washington DC AmeriHealth Formulary
Link
10/2018
Medicaid Drug Utilization Review State Comparison/Summary
Report FFY 2017 Annual Report Prescription Drug Fee-For-
Service Programs
Link
Florida
07/18/2018
Florida PDL
Link
Accessed
08/06/2018
Florida Prior Authorization (Buprenorphine Products [05/24/2017])
Link
03/2014
Florida Behavioral Health Overlay Services
Link
10/2018
Medicaid Drug Utilization Review State Comparison/Summary
Report FFY 2017 Annual Report Prescription Drug Fee-For-
Service Programs
Link
Georgia
08/01/2018
Georgia Medicaid/PeachCare PDL
Link
Accessed
08/08/2018
Georgia Prior Authorization Links
Link
07/01/2018
Georgia Policies and Procedures for Pharmacy Services
Link
08/01/2018
Georgia Amerigroup PDL
Link
07/01/2018
Georgia Policies for Community Behavioral Health &
Rehabilitation Services
Link
Hawaii
Accessed
08/08/2018
Hawaii Fee-for-Service Formulary Drug Search Link
07/01/2018
Hawaii PDL (Hawaii Medical Service Association Blue Cross Blue
Shield)
Link
Accessed
07/16/2018
Hawaii Quest Integration Medical Benefits Link
Idaho
07/01/2018
Idaho PDL
Link
07/01/2018
Idaho Prior Authorization Opioid Dependence Treatments
Link
Illinois
07/01/2018
Illinois PDL
Link
Accessed
08/07/2018
Illinois Prior Authorization Link
08/03/2018
Illinois Formulary (Meridian)
Link
03/2018
Illinois Drugs Billed Under Medical Benefit (Meridian)
Link
01/01/2017
Illinois Fee Schedule for Medication Assisted Treatment
Link
10/2018
Medicaid Drug Utilization Review State Comparison/Summary
Report FFY 2017 Annual Report Prescription Drug Fee-For-
Service Programs
Link
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
71
State
Effective Date
Document Name
Link
Indiana
07/01/2018
Indiana PDL
Link
04/01/2018
Indiana Buprenorphine Products Prior Authorization Criteria
Link
08/01/2018
Indiana MCO PDL
(Anthem Healthy Indiana Basic)
Link
Accessed
08/07/2018
Indiana MCO Formulary (Anthem Healthy Indiana Basic)
Link
07/01/2018
Indiana Outpatient Fee Schedule
Link
07/28/2018
Indiana Professional Fee Schedule
Link
07/14/2018
Indiana Methadone Coverage Document
Link
Iowa
01/01/2017
Iowa PDL Alpha Drug List
Link
06/01/2018
Iowa PDL
Link
06/01/2018
Iowa Methadone Coverage Document
Link
07/01/2018
Iowa Medicaid Prior Authorization Criteria
Link
Kansas
07/01/2018
Kansas PDL
Link
10/12/2016
Kansas Probuphine Prior Authorization
Link
Accessed
07/31/2018
KanCare Amerigroup PDL
Link
07/09/2014
Kansas Criteria for Prior Authorization Opioid Dependence
Agents
Link
01/2017
Kansas Criteria for Non-Preferred Drugs That Require Prior
Authorization
Link
10/2018
Medicaid Drug Utilization Review State Comparison/Summary
Report FFY 2017 Annual Report Prescription Drug Fee-For-
Service Programs
Link
Accessed
07/31/2018
Kaiser Family Foundation States Reporting Medicaid Coverage of
Medication Assisted Treatment (MAT) Drugs
Link
Kentucky
07/09/2018
Kentucky PDL (Magellan)
Link
07/01/2018
Kentucky PDL (WellCare)
Link
02/01/2018
Kentucky Pharmacy Prior Authorization Guidelines (Aetna)
Link
Accessed
07/31/2018
Kentucky Formulary (Passport)
Link
07/21/2018
Kentucky Authorization Requirements (Humana)
Link
Louisiana
10/01/2017
Louisiana Fee-for-Service PDL and Prior Authorization
Link
Accessed
08/01/2018
Louisiana Link to All Medicaid MCO PDLs Link
10/01/2017
Louisiana MCO Common PDL
Link
08/01/2018
Louisiana PDL (Anthem)
Link
Maine
07/28/2018
Maine PDL
Link
01/01/2013
Maine Revised Statutes, title 22-3, § 3174-VV
Link
01/01/2013
Maine Revised Statutes, title 22-3, § 3174-SS
Link
Maryland
07/01/2018
Maryland PDL
Link
07/2018
Maryland Maximum Quantity Listing
Link
07/15/2018
Maryland Vivitrol/Campral Prior Authorization Form
Link
01/01/2015,
updated
10/04/2017
Maryland Clinical Criteria SUD Medications Link
Accessed
08/01/2018
Maryland Pharmacy Program Clinical Criteria Look-up
Link
Accessed
08/01/2018
Maryland MCO Formulary (Amerigroup) Link
08/01/2017
Maryland Community-Based Substance Use Disorder Fee
Schedule
Link
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
72
State
Effective Date
Document Name
Link
Massachusetts
Accessed
08/01/2018
Massachusetts Drug List
Link
07/30/2018
Massachusetts PDL (Drug and Alcohol Agents)
Link
01/2016
Massachusetts Buprenorphine-Naloxone Letter
Link
05/2018
Massachusetts Opioid Dependence and Reversal Agents Prior
Authorization Request
Link
Accessed
07/16/2018
Massachusetts SUD Treatment Manual
Link
Michigan
Accessed
08/02/2018
Michigan Drug Carve Out Link
07/17/2018
Michigan PDL
Link
07/17/2018
Michigan Quantity Limitations
Link
06/01/2018
Michigan Opioid Abuse Treatments
Link
07/2017
Michigan Prior Authorization for Office Based
Opioid Addiction Treatment
Link
07/01/2018
Michigan MCO Common Formulary
Link
2018
Michigan MCO Services Requiring Authorization and Benefit
Exclusions (Molina)
Link
07/01/2018
Michigan Provider Manual (Methadone)
Link
Minnesota
Accessed
08/02/2018
Minnesota Magellan Formulary Look-up
Link
07/18/2018
Minnesota Prior Authorization
Link
02/09/2018
Minnesota Prior Authorization Buprenorphine and
Buprenorphine-Naloxone
Link
02/09/2018
Minnesota Prior Authorization Zubsolv
Link
Accessed
08/02/2018
Minnesota Health Partners Formulary Link
07/10/2017
Minnesota Alcohol and Drug Abuse Services
Link
Mississippi
07/01/2018
Mississippi PDL
Link
01/01/2017
Mississippi Buprenorphine-Naloxone & Buprenorphine Coverage
Link
Accessed
08/01/2018
Mississippi United Healthcare Optum Prescription Drug List
Link
Accessed
07/31/2018
Kaiser Family Foundation States Reporting Medicaid Coverage of
MAT Drugs
Link
Missouri
07/01/2018
Missouri PDL
Link
10/19/2017
Missouri Opioid Dependence Agents PDL
Link
Accessed
08/02/2018
Missouri HealthNet Epocrates
Link
Accessed
07/31/2018
Kaiser Family Foundation States Reporting Medicaid Coverage of
MAT Drugs
Link
Montana
08/02/18
Montana PDL
Link
11/2017
Montana Buprenorphine-Containing Products for Opioid
Substance Use Disorder Prior Authorization
Link
01/01/2018
Montana Fee Schedule Physician Services
Link
Accessed
07/31/2018
Kaiser Family Foundation States Reporting Medicaid Coverage of
MAT Drugs
Link
Nebraska
05/2018
Nebraska PDL
Link
Accessed
08/03/2018
Nebraska Magellan Drug Look-up Link
07/01/2018
Nebraska Summary of Drug Limitations
Link
11/2017
Nebraska Buprenorphine Products Prior Authorization
Link
02/2014
Nebraska Buprenorphine Products Consent
Link
07/01/2018
Nebraska Fee Schedule for Behavioral Health
Link

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
73
State
Effective Date
Document Name
Link
Nevada
02/05/2018
Nevada PDL
Link
11/19/2013
Nevada Prior Authorization Suboxone and Subutex
Link
11/03/2016
Nevada Medicaid Informational Bulletin on Medications and
Services for Substance Use Disorders
Link
08/01/2018
Nevada MCO Preferred Drug List (Anthem)
Link
04/10/2018
Nevada Provider Type 17 Billing Guide
Link
New Hampshire
06/18/2018
New Hampshire PDL
Link
10/08
New Hampshire Quantity Limit Program
Link
Accessed
08/04/2018
New Hampshire WellSense Formulary Link
07/01/2017
WellSense Buprenorphine and Naloxone Products and Wellsense
Sublocade and Wellsense Probuphine Pharmacy Policies
(versions applicable to New Hampshire Medicaid)
Link
Accessed
08/04/2018
New Hampshire Healthy Families Formulary
Link
07/01/2016
New Hampshire SUD Benefit for Standard Medicaid Recipients
Link
New Jersey
07/01/2018
New Jersey PDL (UnitedHealthcare)
Link
Accessed
08/05/2018
New Jersey UnitedHealthcare/Optum PDL Lookup
Link
07/01/2018
New Jersey PDL (WellCare)
Link
04/2018
New Jersey PDL (Horizon)
Link
08/01/2018
New Jersey PDL (Amerigroup)
Link
Accessed
07/16/2018
New Jersey Administrative Code 10:49-5.2(b)(18)
Link
New Mexico
07/01/2018
New Mexico Formulary (Blue Cross Blue Shield)
Link
07/2018
New Mexico Formulary (Molina)
Link
Accessed
08/03/2018
New Mexico Formulary (United Healthcare) Link
Accessed
07/16/2018
New Mexico Fee-for-Service MAT Reimbursement Plan
Link
New York
07/12/2018
New York PDL (Fee for Service)
Link
Accessed
03/17/2017
New York Pharmacy Benefit Information Center
Link
Accessed
03/17/2017
New York Formulary Drug Search (Amerigroup)
Link
05/18/2017
New York State Health and Recovery Plan/Mainstream
Behavioral Health Billing and Coding Manual
Link
North Carolina
08/01/2018
North Carolina PDL
Link
Accessed
07/11/2017
North Carolina Clinical Edits Behavioral Health Adult Link
06/05/2018
North Carolina Prior Approval Buprenorphine &
Buprenorphine/Naloxone
Link
12/2016
North Carolina Medicaid Bulletin
Link
04/01/2017
North Carolina Enhanced Mental Health and Substance Abuse
Services
Link
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
74
State
Effective Date
Document Name
Link
North Dakota
07/01/2018
North Dakota PDL
Link
Accessed
08/11/2018
North Dakota Prior Authorization
Link
Accessed
08/11/2018
North Dakota Department of Human Services Drug Search
Link
Accessed
08/11/2018
North Dakota Opioid Dependence Agents Prior Authorization
Form
Link
Accessed
08/11/2018
North Dakota Naloxone Rescue Agents Prior Authorization Form Link
Ohio
07/01/2018
Ohio PDL (Fee for Service)
Link
Accessed
03/17/2017
Ohio Drug Search Link
06/12/2016
Ohio Quantity Limits
Link
05/2016
Ohio Prior Authorization Form Suboxone/Zubxolv
Link
11/01/2017
OAC 5160-9-03 List of Drugs Covered Without Prior Authorization
Link
08/01/2018
Ohio PDL (Molina)
Link
01/01/2018
Ohio Medicaid Fee Schedule Medicine, Surgery, Radiology and
Imaging, and Additional Procedures
Link
Oklahoma
Accessed
08/13/2018
Oklahoma Pharmacy Central Nervous System/Behavioral Health
Substance Abuse Treatments
Link
Accessed
08/13/2018
Oklahoma Maximum Quantity Limits Link
Accessed
08/13/2018
Oklahoma Prior Authorization WebAlerts
Link
Accessed
07/31/2018
Kaiser Family Foundation States Reporting Medicaid Coverage of
MAT Drugs
Link
Oregon
07/01/2018
Oregon PDL
Link
and
Link
Accessed
08/13/2018
Oregon Health Plan Drug Formulary Link
11/06/2017
Oregon (Family Care) Formulary
Link
02/20/2018
Oregon Health Plan Behavioral Health Fee Schedule
Link
05/30/2018
Oregon Health Plan Fee-for-Service Fee Schedule
Link
Pennsylvania
07/23/2018
Pennsylvania PDL
Link
05/01/2018
Pennsylvania Maximum Quantity Limits
Link
Accessed
08/16/2018
GHS Pennsylvania Fee-for-Service Drug Search
Link
04/10/2018
Pennsylvania Opiate Dependence Treatments Prior Authorization
Form
Link
04/10/2018
Pennsylvania Prior Authorization of Opiate Dependence
Treatments
Link
04/10/2018
Pennsylvania Prior Authorization Probuphine
Link
Accessed
08/16/2018
Pennsylvania Fee-for-Service Billing Information for Naloxone Link
07/18/2016
Pennsylvania Prior Authorization of Opiate Overdose Agents
Link
Accessed
07/27/2018
Pennsylvania Mental Health/Substance Abuse Outpatient Fee
Schedule
Link
Puerto Rico
Accessed
08/06/2018
Puerto Rico Government Health Plan Provider Manual (Molina)
Link
2018
Puerto Rico Salud Mental Formulario de Medicamentos
en Cubierta del PSG
Link
2018
Puerto Rico Salud Fisica Formulario de Medicamentos
en Cubierta del PSG
Link
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
75
State
Effective Date
Document Name
Link
Rhode Island
07/10/2018
Rhode Island PDL
Link
09/29/2017
Rhode Island MCO (Neighborhood Health Plan)
Link
Accessed
07/16/2018
Rhode Island Rehabilitative Services Policy
Link
South Carolina
Accessed
08/16/2018
South Carolina Drug Lookup (Magellan)
Link
08/01/2018
South Carolina PDL (Magellan)
Link
08/01/2018
South Carolina PDL (Blue Cross Blue Shield)
Link
and
Link
06/01/2017
South Carolina Clinical Criteria Buprenorphine Sublingual Tablets
for Medication Assisted Treatment (Blue Cross Blue Shield)
Link
01/09/2018
South Carolina Clinical Criteria Naloxone Agents
(Blue Cross Blue Shield)
Link
Accessed
08/16/2018
South Carolina Formulary Lookup (Blue Cross Blue Shield)
Link
South Dakota
Accessed
08/15/2018
South Dakota Prior Authorization Page
Link
11/04/2014
South Dakota Medications Requiring Prior Authorization
Link
08/02/2011
South Dakota Maximum Units List
Link
Accessed
08/15/2018
South Dakota Evzio Prior Authorization Form
Link
Accessed
08/15/2018
South Dakota Suboxone/Subutex Prior Authorization Form
Link
Accessed
08/15/2018
South Dakota Evzio Prior Authorization Algorithm Link
Accessed
08/15/2018
South Dakota Suboxone/Subutex Prior Authorization Criteria Link
10/2018
Medicaid Drug Utilization Review State Comparison/Summary
Report FFY 2017 Annual Report Prescription Drug Fee-For-
Service Programs
Link
Accessed
07/31/2018
Kaiser Family Foundation States Reporting Medicaid Coverage of
MAT Drugs
Link
Tennessee
08/01/2018
Tennessee PDL (Magellan)
Link
08/01/2018
Tennessee AutoExempt List
Link
07/01/2018
Tennessee Prior Authorization Buprenorphine Products
Link
08/01/2018
Tennessee Clinical Criteria, Step Therapy, and Quantity Limits
(Magellan)
Link
06/26/2018
Naltrexone MAT Program Description Division of TennCare
Link
05/18/2018
Buprenorphine MAT Program Description Division of TennCare
Link
Texas
Accessed
08/15/2018
Texas Formulary Drug Search
Link
07/26/2018
Texas Prior Authorization Criteria
Link
05/2018
Texas Pharmacy Clinical Prior Authorization
Assistance Chart
Link
03/26/2018
Texas Prior Authorization Clinical Criteria
Buprenorphine Agents
Link
Accessed
07/16/2018
Texas Online Fee Lookup Search Link
10/2018
Medicaid Drug Utilization Review State Comparison/Summary
Report FFY 2017 Annual Report Prescription Drug Fee-For-
Service Programs
Link
U.S. Virgin
Islands
N.D.
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
76
State
Effective Date
Document Name
Link
Utah
08/01/2018
Utah PDL
Link
Accessed
08/14/2018
Utah Prior Authorization
Link
08/01/2018
Utah Drug Quantity Limits
Link
07/14/2018
Utah Coverage and Reimbursement Code Look-up
Link
01/12/2018
Utah Prior Authorization Request Oral Buprenorphine and
Buprenorphine/Naloxone Products
Link
02/26/2018
Utah Prior Authorization Request Vivitrol and Sublocade
Link
07/2018
Utah PDL (Molina)
Link
Vermont
07/06/2018
Vermont PDL
Link
Virginia
07/01/2018
Virginia PDL
Link
Accessed
08/15/2018
Virginia Drug Lookup Link
05/31/2017
Virginia Service Authorization Form Oral Buprenorphine Products
Link
Accessed
08/15/2018
Virginia Service Authorization Form Extended Release
Buprenorphine (Sublocade)
Link
12/29/2017
Virginia Addiction and Recovery Treatment Services Manual
Supplement
Link
07/2018
Magellan Virginia Medicaid/
Department of Medical Assistance Services Rates
Link
Washington
07/01/2018
Washington Apple Health PDL
Link
07/01/2018 Washington MAT Coverage Limitation Link
04/01/2018
Washington Substance Use Disorder Billing Guide
Link
West Virginia
07/01/2018
West Virginia Substance Use Disorder Services
Link
07/01/2018
West Virginia PDL
Link
Accessed
08/14/2018
West Virginia Drug Limits
Link
06/11/2018
West Virginia Drug Code List
Link
Accessed
08/14/2018
West Virginia Prior Authorization
Link
07/31/2017
West Virginia Policy for the Coverage of Suboxone Film
(Buprenorphine/Naloxone) and Buprenorphine Tablets
Link
06/01/2018
West Virginia Prior Authorization Criteria Sublocade
(buprenorphine extended-release injection)
Link
Wisconsin
08/01/2018
Wisconsin Medicaid, BadgerCare Plus Standard, and SeniorCare
Preferred Drug List—Quick Reference
Link
08/01/2018
Wisconsin Quantity Limit Drugs
Link
07/2018
Wisconsin Prior Authorization/Preferred Drug List for Opioid
Dependency Agents—Buprenorphine
Link
Accessed
08/12/2018
Wisconsin Drug Search Link
Wyoming
07/17/2018
Wyoming PDL
Link
07/17/2018
Wyoming Dosage Limitation List
Link
07/17/2018
Wyoming Additional Therapeutic Classes with Clinical Criteria
Link
Accessed
08/12/2018
Wyoming Oral Buprenorphine/Naloxone or Oral Buprenorphine
Prior Authorization Request Form
Link
Abbreviations: MAT, medication-assisted treatment; MCO, managed care organization; PDL, Preferred Drug List;
PSG, Plan de Salud del Gobierno de Puerto Rico; SUD, substance use disorder.
Note: Links were current as of August 20, 2018.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
77
Table A-2. Preferred Status of Medications for Alcohol and Opioid Use Disorders or to Reverse
Opioid Overdose Within Medicaid, by State, 2016–2017
a,b,c,d
State
Acamprosate
Disulfiram
Oral Naltrexone
Extended
-
Release
Injectable
Naltrexone
Buprenorphine
Implantable
Buprenorphine
Extended
-
Release
Injectable
Buprenorphine
Buprenorphine
-
Naloxone
Naloxone
Alabama
Yes
Yes
Yes
Yes
No
No
No
Yes
No
Alaska
-
No
Yes
Yes
No
No
-
Yes
No
Arizona
No
No
Yes
Yes
Yes
-
-
Yes
Yes
Arkansas
No
No
No
No
-
No
No
Yes
No
California
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Colorado
Yes
Yes
Yes
No
No
-
No
Yes
Yes
Connecticut
No
-
Yes
Yes
Yes
-
-
Yes
Yes
Delaware
-
No
Yes
Yes
Yes
-
Yes
Yes
Yes
District of
Columbia
- Yes Yes No No No No Yes Yes
Florida
Yes
Yes
Yes
Yes
Yes
-
-
Yes
Yes
Georgia
Yes
Yes
Yes
Yes
Yes
-
-
Yes
Yes
Hawaii
No
Yes
Yes
No
Yes
-
-
Yes
Yes
Idaho
-
No
Yes
Yes
No
No
No
Yes
Yes
Illinois
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Yes
Indiana
Yes
Yes
Yes
No
Yes
-
No
Yes
Yes
Iowa
Yes
Yes
Yes
-
No
-
-
Yes
Yes
Kansas
No
No
No
No
No
No
-
No
No
Kentucky
Yes
Yes
Yes
Yes
Yes
No
No
Yes
Yes
Louisiana
Yes
Yes
Yes
Yes
Yes
No
-
Yes
Yes
Maine
No
Yes
Yes
No
No
No
No
Yes
Yes
Maryland
No
-
Yes
Yes
Yes
-
No
Yes
Yes
Massachusetts
Yes
Yes
Yes
Yes
No
No
No
Yes
Yes
Michigan
Yes
Yes
Yes
Yes
Yes
-
No
Yes
Yes
Minnesota
Yes
Yes
Yes
Yes
Yes
No
No
Yes
Yes
Mississippi
Yes
Yes
Yes
No
Yes
No
No
Yes
Yes
Missouri
-
No
Yes
Yes
No
No
No
Yes
Yes
Montana
No
No
Yes
No
Yes
-
-
Yes
Yes
Nebraska
No
No
Yes
-
No
-
-
Yes
Yes
Nevada
No
Yes
No
No
No
-
-
Yes
Yes
New Hampshire
Yes
Yes
Yes
Yes
Yes
No
No
Yes
Yes
New Jersey
Yes
Yes
Yes
No
Yes
No
-
Yes
Yes
New Mexico
Yes
Yes
Yes
Yes
Yes
No
-
Yes
Yes
New York
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
North Carolina
Yes
Yes
Yes
Yes
No
-
Yes
Yes
Yes
North Dakota
Yes
Yes
No
Yes
No
No
No
Yes
Yes
Ohio
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
Oklahoma
-
-
-
-
No
No
-
Yes
-
Oregon
Yes
Yes
Yes
Yes
Yes
-
-
Yes
Yes
Pennsylvania
-
-
Yes
Yes
Yes
No
No
Yes
Yes
Puerto Rico
-
-
-
-
Yes
-
-
Yes
-
Rhode Island
Yes
Yes
Yes
No
Yes
No
No
Yes
Yes
South Carolina
Yes
Yes
Yes
Yes
Yes
No
Yes
Yes
Yes
South Dakota
-
-
-
-
-
-
-
Yes
No
Tennessee
-
-
Yes
Yes
Yes
-
-
Yes
Yes
Texas
Yes
Yes
Yes
No
Yes
-
-
Yes
Yes
U.S. Virgin
Islands
- - - - - - - - -
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
78
State
Acamprosate
Disulfiram
Oral Naltrexone
Extended
-
Release
Injectable
Naltrexone
Buprenorphine
Implantable
Buprenorphine
Extended
-
Release
Injectable
Buprenorphine
Buprenorphine
-
Naloxone
Naloxone
Utah
-
Yes
Yes
Yes
No
-
No
Yes
-
Vermont
Yes
Yes
Yes
Yes
No
No
No
Yes
Yes
Virginia
-
-
Yes
Yes
Yes
No
No
Yes
Yes
Washington
Yes
Yes
Yes
Yes
No
No
No
Yes
Yes
West Virginia
-
-
No
Yes
No
-
No
Yes
Yes
Wisconsin
No
No
Yes
Yes
No
-
No
Yes
Yes
Wyoming
-
No
Yes
Yes
No
-
-
Yes
No
Totals for All States
Yes
27
32
44
34
29
2
7
51
43
No
11
11
5
13
21
26
24
1
6
Unknown
15
10
4
6
3
25
22
1
4
a
As indicated in the methods section of this report, preferred status may vary between fee-for-service plans and
managed care organizations (MCOs) or among MCOs. If the drug met the criteria for preferred status as laid out in
the methods section of this report, it is identified as preferred in that state.
b
Methadone is covered in Table A-9.
c
Dashes indicate unknown or not applicable for each item.
d
Materials reviewed were those available in the third quarter of 2018.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
79
Table A-3. Medicaid Coverage of Acamprosate, by State, 2016–2017
a,b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
Yes
No
-
No
-
-
Alaska
No
-
-
-
-
-
-
Arizona
Yes
No
No
-
No
-
-
Arkansas
Yes
No
No
-
No
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
Yes
Yes
No
-
No
-
No
Connecticut
No
No
-
-
-
-
-
Delaware
No
-
-
-
-
-
-
District of No - - - - - -
Columbia
Florida
Yes
Yes
No
-
-
-
-
Georgia
Yes
Yes
No
-
Yes
-
-
Hawaii
Yes
No
Yes
-
No
-
-
Idaho
No
-
-
-
-
-
-
Illinois
Yes
Yes
No
-
-
-
-
Indiana
Yes
Yes
No
-
Yes
-
-
Iowa
Yes
Yes
No
-
-
-
-
Kansas
Yes
No
No
-
-
-
-
Kentucky
Yes
Yes
Yes
Yes
Yes
-
Yes
Louisiana
Yes
Yes
No
-
Yes
-
-
Maine
Yes
No
Yes
Yes
-
-
Yes
Maryland
Yes
No
Yes
Yes
Yes
-
Yes
Massachusetts
Yes
Yes
No
-
-
-
-
Michigan
Yes
Yes
Yes
-
No
-
No
Minnesota
Yes
Yes
No
-
Yes
-
-
Mississippi
Yes
Yes
No
-
Yes
-
No
Missouri
Yes
-
-
-
-
-
-
Montana
Yes
No
-
-
-
-
-
Nebraska
Yes
No
-
-
No
-
-
Nevada
Yes
No
Yes
-
Yes
-
-
New Hampshire
Yes
Yes
No
-
No
-
-
New Jersey
Yes
Yes
No
-
Yes
-
-
New Mexico
Yes
Yes
No
-
No
-
No
New York
Yes
Yes
No
-
Yes
-
No
North Carolina
Yes
Yes
No
-
-
-
-
North Dakota
Yes
Yes
No
-
No
-
-
Ohio
Yes
Yes
Yes
-
No
-
-
Oklahoma
No
-
-
-
-
-
-
Oregon
Yes
Yes
Yes
-
Yes
-
No
Pennsylvania
No
-
-
-
-
-
-
Puerto Rico
-
-
Yes
-
-
-
Yes
Rhode Island
Yes
Yes
No
-
No
-
No
South Carolina
Yes
Yes
No
-
Yes
-
No
South Dakota
Yes
-
-
-
-
-
-
Tennessee
No
-
-
-
-
-
-
Texas
Yes
Yes
No
-
-
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
80
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
U.S. Virgin
Islands
-
-
-
-
-
-
-
Utah
No
-
-
-
-
-
-
Vermont
Yes
Yes
No
-
No
-
-
Virginia
Yes
-
No
-
No
-
-
Washington
Yes
Yes
No
-
No
-
-
West Virginia
No
-
-
-
-
-
-
Wisconsin
Yes
No
No
-
No
-
-
Wyoming
No
-
-
-
-
-
-
Totals for All States
Yes
40
27
9
3
12
0
4
No
11
11
28
0
17
0
9
Unknown
2
15
16
50
24
53
40
Abbreviations: FFS, fee-for-service; MCO, managed care organization; PDL, Preferred Drug List.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
Alabama: Only certain brand drugs are identified as preferred and most generics are available without prior
authorization. The formulary identifies this drug as available without authorization or quantity limits.
Arizona: The behavioral health drug list identifies it as not preferred but does not require prior authorization or
quantity limits.
Arkansas: The drug is available without limits on formulary. Preferred status is indicated as “N/A."
California: Acamprosate is preferred on the Health and Wellness PDL.
Colorado: The drug is not on the FFS drug list, but neither is this drug class. It is included as preferred on a selected
MCO formulary without prior authorization or other limits.
Connecticut: The drug is not on the PDL, and there is no evidence of reimbursement in the CMS data.
Delaware: The drug is not on the FFS PDL and is nonformulary on MCO, with no evidence of any reimbursement in
2018.
District of Columbia: The drug is not on the FFS PDL or MCO formulary, and no reimbursement is found in 2018.
Hawaii: The drug is not on the FFS formulary. The generic is covered with prior authorization on a selected MCO
formulary.
Illinois: Generic is preferred.
Indiana: Neutral drugs (including acamprosate) are not listed on the FFS PDL and are neither preferred nor
nonpreferred. The drug is preferred with quantity limits for the selected MCO.
Iowa: Generic is preferred with no prior authorization.
Kentucky: The FFS PDL does not identify this as preferred, but at least one MCO does identify it as preferred,
whereas another requires prior authorization and imposes quantity limits, step therapy, and participation in a
treatment program or recovery plan.
Louisiana: The drug is not listed on the FFS PDL or the MCO common PDL. At least one MCO identifies it as
preferred with quantity limits.
Maine: The drug is nonpreferred on the FFS PDL and prior authorization is required. It is required to be used as part
of a “formal structured outpatient detoxification program,” and preferred drugs must be tried first.
Maryland: The drug class is not on the FFS PDL, but the program does impose quantity limits, requirements for prior
authorization, counseling, and failure of preferred drugs required.
Michigan: FFS does not list the drug. It is preferred on the common MCO formulary without prior authorization.
There are no quantity limits for FFS or the MCO formulary. The carve-out requires prior authorization.
Mississippi: The FFS PDL does not include the drug, but at least one MCO treats it as preferred.
Missouri: The state uses Epocrates for its formulary. Epocrates identifies it as covered. No other information is
available.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
81
Montana: The drug is not on the FFS PDL, and there is no indication regarding requirements for prior authorization.
Nebraska: The drug is listed on formulary as covered for some but not all doses. The drug class is not on the PDL
and requirements for prior authorization are unclear.
Nevada: The general FFS PDL does not list the drug. Substance use specific information (dated in that MCOs have
since changed) indicated prior authorization is required for some and that quantity limits are required for some.
New Hampshire: The drug class is not on the FFS PDL, and it is not listed in one MCO formulary. One MCO
formulary lists it as generic preferred. It is not listed on the FFS quantity limits document (which is dated).
New Jersey: Generic is preferred by a selected MCO, with quantity limits.
New York: The drug class is not on the FFS PDL. It is preferred on most MCO formularies, and for the selected
plan, there is no prior authorization, but quantity limits are imposed.
North Carolina: The state treats the drug as preferred as the class is not listed. Prior authorization is not required.
North Dakota: Only certain classes are on PDL; this class is not included. Drug look-up indicates it is covered
without prior authorization or quantity limits; treating as preferred.
Ohio: The drug is on the state FFS formulary, but the drug class is not on the PDL; there is no prior authorization and
no quantity limit under FFS for generic or brand. The selected MCO identifies it as preferred with prior authorization.
Oklahoma: There is no evidence of coverage.
Oregon: Generic is preferred without prior authorization for FFS. The Oregon Health Plan drug list requires prior
authorization and quantity limits.
Pennsylvania: The class is not on the FFS PDL, and it is not included in the current FFS formulary and has not been
reimbursed in 2018.
Puerto Rico: The drug class is not included in the mental health formulary, although detoxification medications are
included. Use of any drug not in the formulary requires approval and a showing of failure or other reasons why
formulary medications cannot be used.
Rhode Island: The drug class is not listed on the FFS PDL, but generic is included without prior authorization or
other limits on one MCO formulary.
South Carolina: This drug class is not on the FFS PDL. The generic is on the FFS formulary without prior
authorization or other limits. The selected MCO identifies it as preferred with quantity limits.
South Dakota: The state seemingly does not have a publicly available PDL. There is no evidence of coverage in
other pharmacy documents nor evidence of reimbursement in the 2018 CMS data.
Tennessee: The class is not on the state PDL, there were no reimbursements in the CMS data, and there is no
publicly available information indicating it is covered.
Utah: This class is not included on the PDL, and there were no reimbursements in the 2018 CMS data.
Virginia: This class is not covered by the PDL. The drug look-up does not include a service authorization or quantity
limit requirement.
Washington: Generic is preferred without limits. There are clinical cautions for acamprosate.
West Virginia: This class is not managed under the PDL. Documents indicate nonmanaged classes are covered as
they historically have been. There was no reimbursement in the CMS 2018 data.
Wisconsin: Generic is on the formulary (without prior authorization) but not the PDL. The drug class is included on
the PDL, but acamprosate is not.
Wyoming: There is no evidence of coverage of this drug.
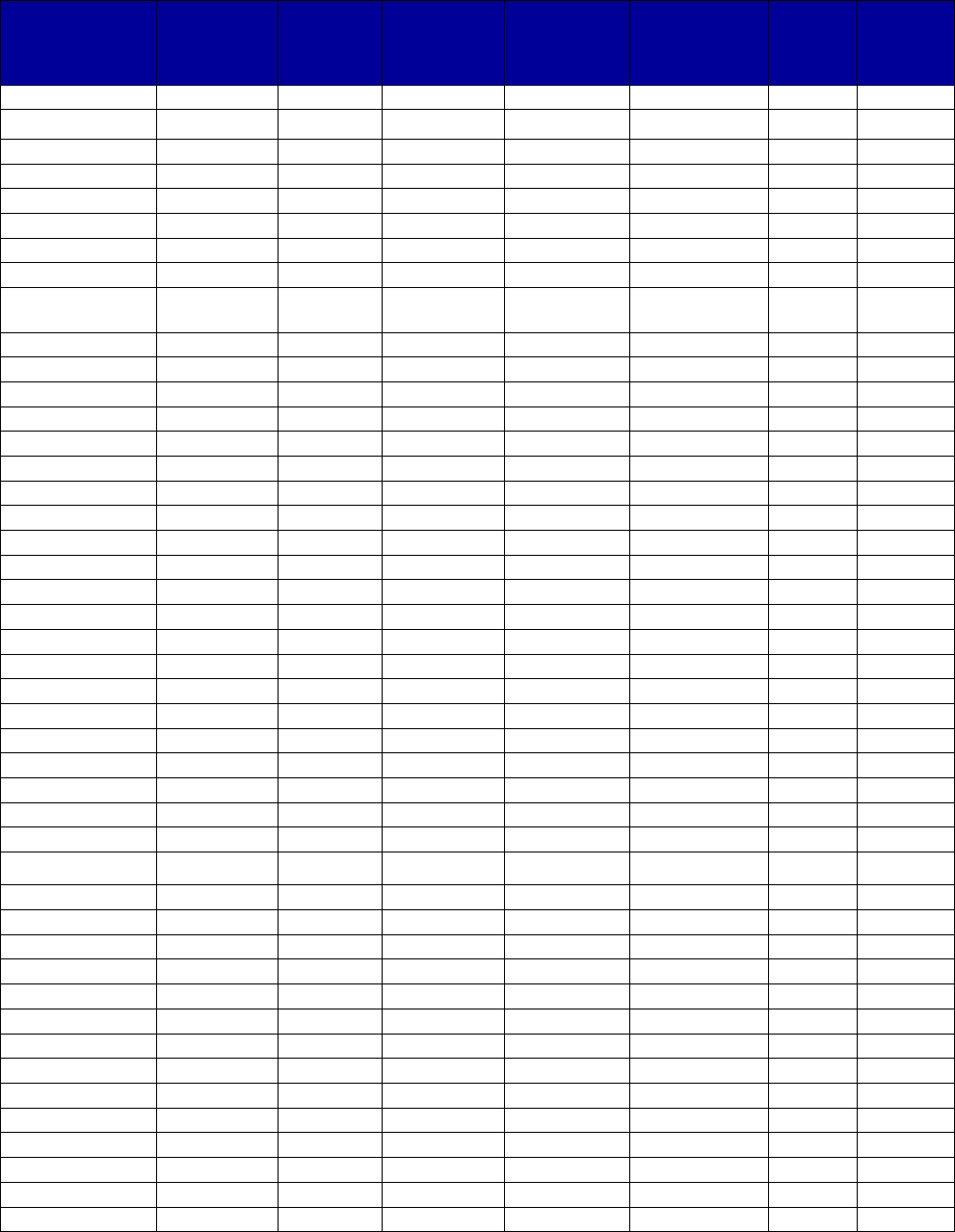
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
82
Table A-4. Medicaid Coverage of Disulfiram, by State, 2016–2017
a,b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
Yes
No
-
No
-
-
Alaska
Yes No - - - - -
Arizona
Yes
No
No
-
No
-
-
Arkansas
Yes
No
No
-
No
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
Yes
Yes
No
-
No
-
No
Connecticut
Yes
-
-
-
-
-
-
Delaware
Yes
No
-
-
-
-
-
District of Yes Yes No - - - -
Columbia
Florida
Yes
Yes
No
-
-
-
-
Georgia
Yes
Yes
No
-
Yes
-
-
Hawaii
Yes
Yes
No
-
No
-
-
Idaho
Yes
No
-
-
-
-
-
Illinois
Yes
Yes
No
-
-
-
-
Indiana
Yes
Yes
No
-
No
-
-
Iowa
Yes
Yes
No
-
-
-
-
Kansas
No
No
No
-
-
-
-
Kentucky
Yes
Yes
-
-
-
-
-
Louisiana
Yes
Yes
No
-
No
-
-
Maine
Yes
Yes
No
No
-
-
No
Maryland
Yes
-
-
-
-
-
-
Massachusetts
Yes
Yes
No
-
-
-
-
Michigan
Yes
Yes
No
-
No
-
No
Minnesota
Yes
Yes
No
-
No
-
-
Mississippi
Yes
Yes
No
-
Yes
-
No
Missouri
Yes
No
-
-
-
-
-
Montana
Yes
No
-
-
-
-
-
Nebraska
Yes
No
-
-
No
-
-
Nevada
Yes
Yes
No
-
No
-
-
New Hampshire
Yes
Yes
No
-
No
-
-
New Jersey
Yes
Yes
No
-
No
-
No
New Mexico
Yes
Yes
No
-
No
-
No
New York
Yes
Yes
No
-
No
-
No
North Carolina
Yes
Yes
No
-
-
-
-
North Dakota
Yes
Yes
No
-
No
-
-
Ohio
Yes
Yes
No
-
Yes
-
-
Oklahoma
Yes
-
-
-
-
-
-
Oregon
Yes
Yes
No
-
No
-
No
Pennsylvania
Yes
-
-
-
-
-
-
Puerto Rico
-
-
Yes
-
-
-
Yes
Rhode Island
No
Yes
No
-
No
-
No
South Carolina
Yes
Yes
No
-
No
-
No
South Dakota
Yes
-
-
-
-
-
-
Tennessee
Yes
-
-
-
-
-
-
Texas
Yes
Yes
No
-
-
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
83
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
U.S. Virgin
Islands
-
-
-
-
-
-
-
Utah
Yes
Yes
No
-
No
-
-
Vermont
Yes
Yes
No
-
No
-
-
Virginia
Yes
-
No
-
No
-
-
Washington
Yes
Yes
No
-
No
-
-
West Virginia
Yes
-
-
-
-
-
-
Wisconsin
Yes
No
No
-
No
-
-
Wyoming
Yes
No
-
-
-
-
-
Totals for All States
Yes
49
32
1
0
3
0
1
No
2
11
36
1
25
0
11
Unknown
2
10
16
52
25
53
41
Abbreviations: FFS, fee-for-service; MCO, managed care organization; PDL, Preferred Drug List.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
Alabama: Only certain brand drugs are identified as preferred, and most generics are available without prior
authorization. The formulary identifies this drug as available without authorization or quantity limits.
Arizona: The behavioral health drug list identifies it as not preferred but does not require prior authorization or
quantity limits.
Arkansas: The drug is available without limits on formulary. Preferred status is indicated as “N/A."
California: The generic is preferred on the Health and Wellness PDL.
Colorado: The drug is not on the FFS drug list, but neither is this drug class. It is included as preferred on a selected
MCO formulary without prior authorization or other limits.
Connecticut: The drug is not on the PDL but is shown as reimbursed in the CMS data.
Delaware: The drug is not on the FFS PDL; the generic drug is included on an MCO formulary as “supplemental.”
District of Columbia: The drug is not on the FFS PDL, but the generic is included on MCO formulary without
restrictions.
Idaho: The drug is not listed in the PDL, but the CMS Medicaid drug data do indicate it has been reimbursed in 2018.
Illinois: The generic and brand are preferred on the FFS PDL.
Indiana: Neutral drugs (including disulfiram) are not listed on the FFS PDL and are neither preferred nor
nonpreferred. The selected MCO treats the generic as preferred and does not require prior authorization.
Iowa: Generic is preferred; brand is not.
Kentucky: Identified as preferred by some plans and not by others.
Louisiana: This is not on the FFS or common MCO PDL, but at least one MCO identifies it as preferred without prior
authorization or quantity limits.
Maine: Both generic and brand drugs are preferred.
Maryland: The FFS PDL is not applicable to this class. It is treated as preferred in that it is one of the two drugs that
must be tried and failed before acamprosate can be reimbursed. At least one MCO includes it on its formulary and
does not require prior authorization.
Minnesota: The generic is preferred without prior authorization or quantity limits.
Mississippi: The FFS PDL does not include the drug, but at least one MCO treats it as preferred.
Missouri: Missouri HealthNet uses Epocrates as its formulary, and it indicates the drug is not covered by Missouri
Medicaid. It was, however, reimbursed by Missouri Medicaid in 2018.
Nebraska: The drug class is not on the PDL, but it is listed on formulary. Prior authorization requirements are
unclear.
Nevada: The drug is not listed on the FFS PDL; however, it is included on the state Medicaid substance use
treatment document as not requiring prior authorization.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
84
New Hampshire: The generic is preferred on one MCO PDL. It is not on the FFS PDL or quantity limits list.
New Jersey: The drug is preferred by a selected MCO.
New York: The drug class is not on the FFS PDL. It is preferred on most MCO formularies, and for the selected
plan, there is no prior authorization, but quantity limits are imposed.
North Carolina: The state treats the drug as preferred as the class is not listed. Prior authorization is not required.
North Dakota: Only certain classes are on the PDL; this class is not included. Drug look-up indicates that tablets are
covered without prior authorization or quantity limits; treating as preferred.
Ohio: The drug class is not on the FFS PDL, but the generic is covered without prior authorization. Quantity limits
apply. The selected MCO identifies it as preferred.
Oklahoma: Disulfiram was reimbursed in 2018.
Oregon: The drug is not on the FFS PDL. The Oregon Health Plan formulary preferred generic without prior
authorization or quantity limits.
Pennsylvania: The drug class is not subject to the FFS PDL, but it is not included on the current formulary for the
FFS program. It has, however, been reimbursed in 2018.
Puerto Rico: The drug class is not included in the mental health formulary, although detoxification medications are
included. Use of any drug not in the formulary requires approval and a showing of failure or other reasons why
formulary medications cannot be used.
Rhode Island: The drug class is not on the FFS PDL. A selected MCO prefers generic without prior authorization or
other limits.
South Carolina: This drug class is not on the FFS PDL. The generic is on the FFS formulary without prior
authorization or other limits. The selected MCO identifies it as preferred.
South Dakota: The state seemingly does not have a publicly available PDL. There is no evidence of coverage in
other pharmacy documents, but there was reimbursement shown in the 2018 CMS data.
Tennessee: The class is not on the state PDL, but there were 2018 reimbursements in the CMS data.
Utah: This class is not on the FFS PDL, but disulfiram is included as preferred on the selected MCO PDL.
Vermont: Disulfiram is preferred without prior authorization. Brand is nonpreferred and requires prior authorization
and proof of intolerance of generic.
Virginia: This class is not covered by the PDL. The drug look-up does not include a service authorization or quantity
limit requirement.
Washington: Generic is preferred without limits.
West Virginia: This class is not managed under the PDL. Documents indicate nonmanaged classes are covered as
they historically have been. There is evidence that the drug was reimbursed in 2018.
Wisconsin: Generic is on the formulary (without prior authorization) but not the PDL. The drug class is included on
the PDL, but disulfiram is not.
Wyoming: Other drugs are identified as preferred or nonpreferred in chemical dependency class, including for
alcohol dependence. Disulfiram and Antabuse are not mentioned. Disulfiram was reimbursed in 2018 in Wyoming.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
85
Table A-5. Medicaid Coverage of Naltrexone, by State, 2016–2017
a,b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
Yes
No
-
No
-
-
Alaska
Yes Yes No - No - -
Arizona
Yes
Yes
No
-
No
-
-
Arkansas
Yes
No
Yes
-
Yes
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
Yes
Yes
No
-
No
-
-
Connecticut
Yes
Yes
No
-
-
-
-
Delaware
Yes
Yes
No
-
No
-
No
District of
Yes
Yes
No
-
-
-
-
Columbia
Florida
Yes
Yes
No
-
-
-
-
Georgia
Yes
Yes
No
-
No
-
-
Hawaii
Yes
Yes
No
-
No
-
-
Idaho
Yes
Yes
Yes
-
-
-
-
Illinois
Yes
Yes
No
-
-
-
-
Indiana
Yes
Yes
No
-
No
-
-
Iowa
Yes
Yes
No
-
-
-
-
Kansas
Yes
No
No
-
-
-
-
Kentucky
Yes
Yes
No
-
No
-
-
Louisiana
Yes
Yes
No
-
No
-
-
Maine
Yes
Yes
No
-
No
-
-
Maryland
Yes
Yes
No
c
No
No
-
-
Massachusetts
Yes
Yes
No
-
-
-
-
Michigan
Yes
Yes
No
-
No
-
No
Minnesota
Yes
Yes
No
-
No
-
-
Mississippi
Yes
Yes
No
-
No
-
No
Missouri
Yes
Yes
Yes
-
Yes
-
-
Montana
Yes
Yes
-
-
-
-
-
Nebraska
Yes
Yes
No
-
No
-
-
Nevada
Yes
No
Yes
-
No
-
-
New Hampshire
Yes
Yes
Yes
-
No
-
-
New Jersey
Yes
Yes
No
-
No
-
No
New Mexico
Yes
Yes
No
-
No
-
No
New York
Yes
Yes
No
-
No
-
No
North Carolina
Yes
Yes
No
-
-
-
-
North Dakota
Yes
No
Yes
-
Yes
-
-
Ohio
Yes
Yes
No
-
No
-
No
Oklahoma
Yes
-
-
-
-
-
-
Oregon
Yes
Yes
No
-
No
-
No
Pennsylvania
Yes
Yes
No
-
No
-
-
Puerto Rico
-
-
Yes
-
-
-
Yes
Rhode Island
Yes
Yes
No
-
No
-
No
South Carolina
Yes
Yes
No
-
No
-
No
South Dakota
Yes
-
-
-
-
-
-
Tennessee
Yes
Yes
Yes
Yes
No
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
86
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Texas
Yes
Yes
No
-
-
-
-
U.S. Virgin
Islands
- - - - - - -
Utah
Yes
Yes
No
-
Yes
-
No
Vermont
Yes
Yes
No
-
No
-
-
Virginia
Yes
Yes
No
-
No
-
No
Washington
Yes
Yes
No
c
-
No
-
-
West Virginia
Yes
No
-
-
Yes
-
-
Wisconsin
Yes
Yes
No
-
No
-
-
Wyoming
Yes
Yes
No
-
No
-
-
Totals for All States
Yes
51
44
8
1
5
0
1
No
0
5
40
1
33
0
13
Unknown
2
4
5
51
15
53
39
Abbreviations: FFS, fee-for-service; MCO, managed care organization; PDL, Preferred Drug List.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
c
See state information below.
Alabama: Only certain brand drugs are identified as preferred, and most generics are available without prior
authorization. The formulary identifies this drug as available without authorization or quantity limits.
Arizona: The behavioral health drug list identifies it as preferred with no prior authorization or quantity limits.
Arkansas: The drug is available without limits on formulary. Preferred status is indicated as “N/A."
California: Naltrexone is preferred on the Health and Wellness PDL without limits.
Colorado: Generic and brand drugs are on the FFS prior authorization and quantity limits document without either
prior authorization or quantity limits.
Georgia: The drug is not listed on the FFS PDL but is preferred without authorization by the selected PDL.
Hawaii: The drug is not listed on the FFS formulary but is preferred without prior authorization for the selected MCO.
Idaho: Generic is preferred with prior authorization.
Illinois: The drug is preferred without prior authorization.
Indiana: The drug is not on the FFS PDL, but it only has limited classes. The tablet formulation is preferred on at
least one MCO formulary with no limits.
Kentucky: The drug is preferred for multiple state Medicaid plans.
Louisiana: FFS lists the generic drug as preferred for opioid dependence but does not list alcohol dependence
treatments. At least one MCO identifies it as preferred for opioid dependence treatment.
Maine: Generic is preferred for both alcohol and opioid dependence treatment.
Maryland: Generic and brand drugs are preferred. Clinical criteria include a requirement for prior authorization if
there was a paid claim within the previous 35 days for bupropion hydrochloride extended-release tablets or bupropion
hydrochloride.
Massachusetts: Generic tablet does not require prior authorization.
Missouri: Naltrexone is preferred. Although it appears that prior authorization is not required by the general PDL,
materials specific to treatment of opioid use disorder suggest otherwise.
Nebraska: Naltrexone is preferred on the PDL but is listed in the opioid reversal drug class.
Nevada: The drug is not on the FFS PDL, although the class is. The state Medicaid substance use specific
document indicates clinical prior authorization is required for FFS. The MCO identified on that document as requiring
quantity limits is no longer an MCO in the state.
New Hampshire: The FFS PDL does not list naltrexone in the opioid dependence treatment class, but it imposes a
comprehensive prior authorization requirement on the entire class without regard to preferred status. A selected
MCO prefers naltrexone without limit or prior authorization.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
87
New Jersey: The drug is preferred by a selected MCO.
New York: The drug is preferred on the FFS PDL without limits. It is covered by the MCOs, and the selected MCO
imposes no limits.
North Dakota: The drug is not on the PDL but is on the Department of Human Services drug look-up with prior
authorization and quantity limit requirements; treating as nonpreferred.
Ohio: The drug is not on the FFS PDL, but it is listed on the drug look-up as being available without prior
authorization, as well as being on the list of drugs, listed by statutory requirement, that do not require prior
authorization. The selected MCO identifies it as preferred.
Oklahoma: The drug was reimbursed in 2018.
Puerto Rico: The drug is not included in the mental health formulary, although opioid treatment medications are
included. Use of any drug not in the formulary requires approval and a showing of failure or other reasons why
formulary medications cannot be used.
South Carolina: The drug is not on the FFS PDL, although Vivitrol is; however, the FFS formulary lists it as being
available without limits. The selected MCO PDL identifies it as a preferred treatment.
South Dakota: The state seemingly does not have a publicly available PDL. There is no evidence of coverage in
other pharmacy documents although it was reimbursed by Medicaid in 2018.
Tennessee: Naltrexone is preferred but requires prior authorization and counseling.
Utah: The drug is preferred on the FFS PDL and quantity limits are imposed. Molina includes it on formulary without
prior authorization, quantity limits, or step therapy.
Washington: Generic is preferred without limits for patients older than 18 years. There are clinical cautions and
restrictions (e.g., past 3 days for opioid withdrawal; if the patient is pregnant or has decompensated liver disease).
West Virginia: Naltrexone is not listed on the PDL, although Vivitrol is (see Table A-6). Injectable naltrexone, short
and long-acting, is on the drug code list with quantity limits.
Wisconsin: Prior authorization is required only if diagnostic criteria are not met.
Wyoming: Clinical criteria apply if certain other medications are to be prescribed (narcotic, carisoprodol,
benzodiazepines), and diagnostic criteria apply.
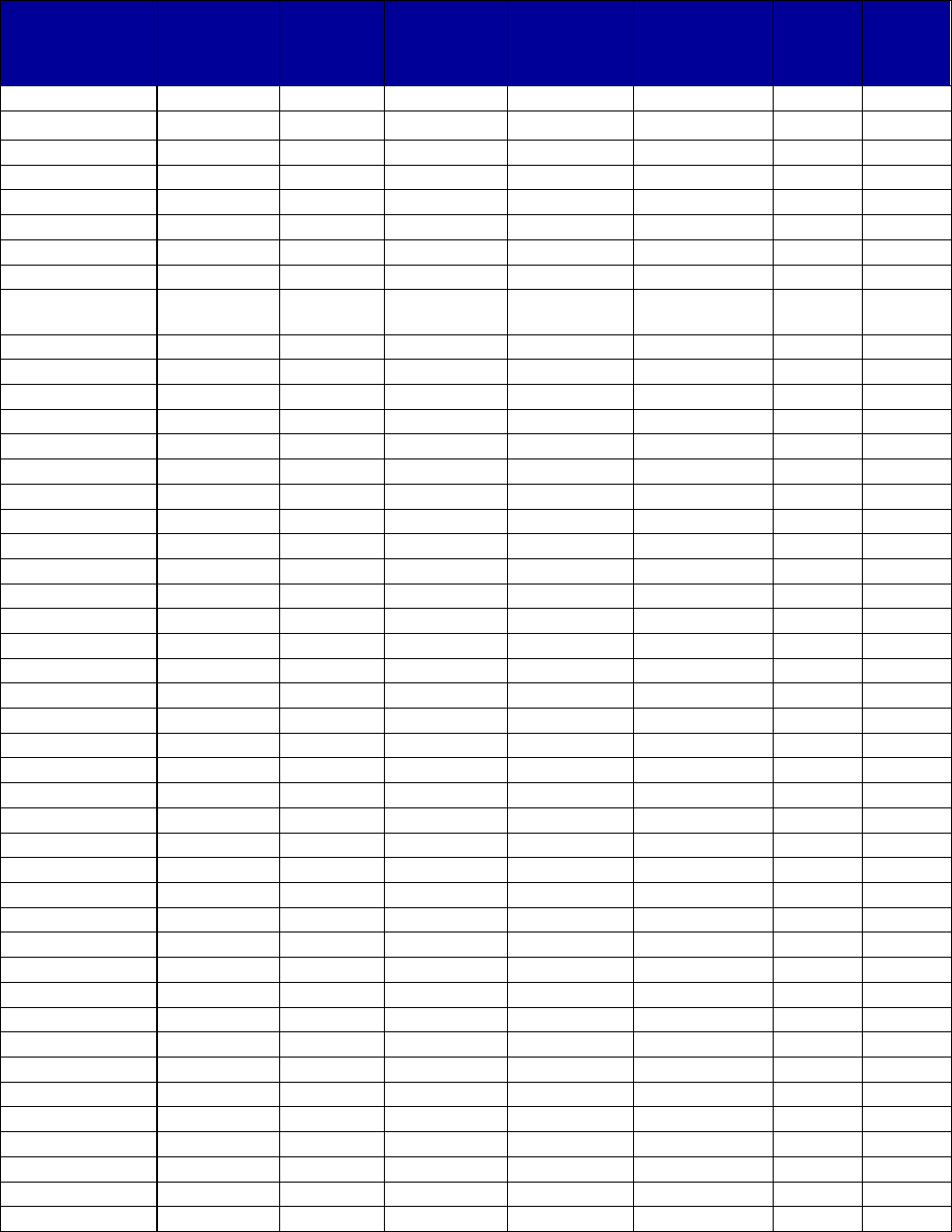
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
88
Table A-6. Medicaid Coverage of Extended Release Injectable Naltrexone, by State, 2016–2017
a,b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
Yes
No
-
No
-
-
Alaska Yes Yes No - No - -
Arizona
Yes
Yes
No
-
No
-
-
Arkansas
Yes
No
-
-
-
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
Yes
No
Yes
-
No
-
-
Connecticut
Yes
Yes
No
-
-
-
-
Delaware
Yes
Yes
Yes
Yes
Yes
-
Yes
District of
Yes
No
Yes
-
Yes
-
-
Columbia
Florida
Yes
Yes
Yes
-
-
-
-
Georgia
Yes
Yes
No
-
Yes
-
-
Hawaii
Yes
No
-
-
-
-
-
Idaho
Yes
Yes
Yes
-
-
-
-
Illinois
Yes
Yes
No
-
-
-
-
Indiana
Yes
No
No
-
-
-
-
Iowa
Yes
-
-
-
-
-
-
Kansas
Yes
No
No
-
-
-
-
Kentucky
Yes
Yes
Yes
Yes
Yes
-
Yes
Louisiana
Yes
Yes
Yes
-
Yes
-
-
Maine
Yes
No
No
-
-
-
-
Maryland
Yes
Yes
Yes
Yes
Yes
-
-
Massachusetts
Yes
Yes
No
-
-
-
-
Michigan
Yes
Yes
No
-
No
-
No
Minnesota
Yes
Yes
Yes
-
Yes
-
-
Mississippi
Yes
No
Yes
-
Yes
-
-
Missouri
Yes
Yes
Yes
No
Yes
-
-
Montana
Yes
No
Yes
-
-
-
-
Nebraska
Yes
c
-
-
-
-
-
-
Nevada
Yes
No
Yes
-
Yes
-
-
New Hampshire
Yes
Yes
No
-
No
-
-
New Jersey
Yes
No
-
-
-
-
-
New Mexico
Yes
Yes
No
-
Yes
-
No
New York
Yes
Yes
No
-
-
-
Yes
North Carolina
Yes
Yes
No
-
Yes
-
-
North Dakota
Yes
Yes
No
-
Yes
-
-
Ohio
Yes
Yes
Yes
c
-
No
-
-
Oklahoma
Yes
-
-
-
-
-
-
Oregon
Yes
Yes
c
No
-
No
-
-
Pennsylvania
Yes
Yes
No
c
No
c
Yes
-
-
Puerto Rico
-
-
Yes
-
-
-
Yes
Rhode Island
Yes
No
Yes
-
No
-
No
South Carolina
Yes
Yes
No
c
-
No
-
-
South Dakota
Yes
-
-
-
-
-
-
Tennessee
Yes
Yes
Yes
Yes
-
-
-
Texas
Yes
No
Yes
-
-
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
89
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
U.S. Virgin
Islands
-
-
-
-
-
-
-
Utah
Yes
Yes
Yes
Yes
-
-
-
Vermont
Yes
Yes
c
No
-
Yes
-
-
Virginia
Yes
Yes
No
-
No
-
No
Washington
Yes
Yes
No
-
No
-
-
West Virginia
Yes
Yes
No
-
Yes
-
-
Wisconsin
Yes
Yes
No
-
No
-
-
Wyoming
Yes
Yes
No
-
No
-
-
Totals for All States
Yes
51
34
19
5
16
0
4
No
0
13
26
2
15
0
5
Unknown
2
6
8
46
22
53
44
Abbreviations: FFS, fee-for-service; MAT, medication-assisted treatment; MCO, managed care organization; PDL,
Preferred Drug List.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
c
See state information below.
Alabama: The formulary identifies this drug as available without authorization or quantity limits.
Arizona: The behavioral health drug list identifies it as preferred with no prior authorization or quantity limits.
Arkansas: Vivitrol is listed on the PDL as neither preferred nor nonpreferred but, rather, as a medical benefit.
California: The drug is preferred without limit on the Health and Wellness PDL.
Colorado: The drug is not on the FFS PDL but is listed on the FFS quantity limits and prior authorization guidance,
where it states that approval will be granted if administered in the patient’s home or in a long-term care facility.
Otherwise, it must be billed as a medical expense.
Delaware: The drug is preferred on the FFS PDL but requires prior authorization, counseling, quantity limits, and an
“oral naltrexone challenge.”
District of Columbia: The drug is identified as not preferred on the FFS PDL and requires prior authorization. A
selected MCO includes it on its formulary with quantity limits.
Florida: The drug is preferred and, on the FFS PDL, has an “auto-PA” status, meaning that the system will
automatically approve if certain criteria are clear at the point of entry.
Georgia: The drug is preferred with FFS without prior authorization but with quantity limits.
Hawaii: The drug is not listed on the FFS formulary or the selected MCO formulary, but a small quantity was
reimbursed in 2018.
Idaho: The drug is preferred for treatment of both alcohol and opioid use disorders.
Illinois: The drug is preferred without prior authorization on the FFS PDL.
Indiana: The drug is not on the FFS PDL, but it only has limited classes, and classes not included are neutral
regarding preference. It is listed as nonpreferred on at least one MCO with no prior authorization.
Iowa: Coverage of extended-release injectable naltrexone is not found in state Medicaid documents, but it was
reimbursed in 2018 in the Medicaid drug utilization data.
Kentucky: The drug is preferred on some Medicaid plans but not others. For nonpreferred, prior authorization is
required, and for at least one managed care plan, step therapy, treatment participation, and quantity limits are
imposed.
Louisiana: The FFS PDL identifies it as not preferred and requires prior authorization. At least one MCO lists it on
its PDL and imposes quantity limits but not prior authorization.
Maine: The drug is preferred without prior authorization.
Maryland: The drug is preferred, but prior authorization, counseling, and quantity limits are required, as well as
satisfaction of additional clinical criteria.
Massachusetts: The drug has no requirement for prior authorization.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
90
Michigan: On the common MCO PDL, the drug appears to be preferred only for alcohol treatment and not opioid
treatment. No distinction is made on the carve-out formulary.
Minnesota: There are prior authorization and quantity limits on extended-release injectable naltrexone.
Missouri: Vivitrol is preferred, and the general PDL indicates no prior authorization is required for preferred drugs;
however, the PDL specific to MAT suggests otherwise.
Montana: The drug is not on the FFS PDL, although generic naltrexone is.
Nebraska: The state formulary identifies the drug as not covered, but it is covered as a medical service.
Nevada: The drug is not on the FFS PDL. The state Medicaid substance use treatment reimbursement guide
indicates that prior authorization and quantity limits apply.
New Hampshire: The FFS PDL does not list Vivitrol in the opioid dependence treatment class, but it imposes a
comprehensive prior authorization requirement on the entire class without regard to preferred status. A selected
MCO lists Vivitrol as Tier 2 (treated here as nonpreferred) without limit or prior authorization.
New Jersey: Three selected MCOs do not include the drug on their PDLs. A fourth MCO does include it on its
formulary with ambiguous limitations that may include prior authorization.
New Mexico: The drug is not on either of two selected MCO PDLs. It is on the formulary of a third MCO without prior
authorization or step therapy, although quantity limits are imposed (treated as preferred).
New York: The drug is preferred on the FFS PDL, and it is covered by all MCOs. The selected MCO does not
require prior authorization of quantity limits but does impose step therapy.
Ohio: The drug is preferred in the FFS program without prior authorization, although there are quantity limits. The
selected MCO does not include Vivitrol on its PDL, indicating that prior authorization would be required.
Oklahoma: The drug was reimbursed in 2018.
Oregon: The drug is preferred without authorization on the FFS PDL but is not on the Oregon Health Plan formulary,
although it is included as a medical benefit for that plan.
Pennsylvania: The drug is preferred with quantity limits. The current FFS PDL indicates that prior authorization is
required, but the April 2018 prior authorization guidance for opioid treatment indicates that is no longer true.
Counseling participation is a prerequisite for prior authorization, if that were to be required.
Puerto Rico: The drug is not included in the mental health formulary, although opioid treatment medications are
included. Use of any drug not in the formulary requires approval and a showing of failure or other reasons why
formulary medications cannot be used.
South Carolina: The drug is preferred without limits on the FFS PDL and formulary. The selected MCO treats it as
nonpreferred and a medical benefit.
South Dakota: The state seemingly does not have a publicly available PDL. There is no evidence of coverage in
other pharmacy documents, although it was reimbursed by Medicaid in 2018.
Tennessee: The drug is not listed on the PDL, although generic and brand naltrexone are. There was
reimbursement during 2018, and it is identified as preferred, with counseling required, by a TennCare document
applicable to the three MCOs.
Utah: The drug is preferred on the FFS PDL but is not on the selected MCO PDL. In FFS, clinical prior authorization
is required with documentation of psychosocial services.
Vermont: The drug is preferred, if clinical criteria are met, for treatment of alcohol dependence and for prevention of
relapse in opioid dependence treatment. Quantity limits apply to both.
Virginia: Extended-release injectable naltrexone is preferred without either prior authorization or quantity limits.
Washington: Extended-release injectable naltrexone is preferred without limits. There are several clinical
prescribing guidelines that explicitly are not requirements for authorization.
West Virginia: The drug is preferred on the PDL with no prior authorization quantity limits on the drug code list.
Wisconsin: Prior authorization is required only if diagnostic criteria are not met.
Wyoming: Clinical criteria apply if certain other medications are to be prescribed (narcotic, carisoprodol,
benzodiazepines), and diagnostic criteria apply.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
91
Table A-7. Medicaid Coverage of Buprenorphine, by State, 2016–2017
a,b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
No
Yes
-
Yes
-
-
Alaska Yes No Yes - Yes - -
Arizona
Yes
Yes
c
No
c
-
No
-
-
Arkansas
Yes
-
c
Yes
Yes
Yes
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
Yes
No
Yes
-
Yes
-
-
Connecticut
Yes
Yes
No
-
Yes
-
-
Delaware
Yes
Yes
No
-
Yes
-
No
District of
Yes
No
Yes
-
Yes
-
-
Columbia
Florida
Yes
Yes
Yes
Yes
Yes
-
-
Georgia
Yes
Yes
No
-
Yes
-
-
Hawaii
Yes
Yes
No
-
No
-
-
Idaho
Yes
No
Yes
-
Yes
-
Yes
Illinois
Yes
Yes
No
-
Yes
-
-
Indiana
Yes
Yes
Yes
Yes
Yes
-
-
Iowa
Yes
No
Yes
Yes
Yes
-
-
Kansas
Yes
No
Yes
Yes
c
Yes
-
-
Kentucky
Yes
Yes
Yes
Yes
Yes
-
-
Louisiana
Yes
Yes
Yes
-
Yes
-
-
Maine
Yes
No
Yes
Yes
Yes
No
c
Yes
Maryland
Yes
Yes
No
-
Yes
-
-
Massachusetts
Yes
No
Yes
-
No
-
-
Michigan
Yes
Yes
Yes
Yes
Yes
No
No
Minnesota
Yes
Yes
Yes
-
Yes
-
-
Mississippi
Yes
Yes
No
-
Yes
-
No
Missouri
Yes
No
Yes
-
Yes
-
-
Montana
Yes
Yes
Yes
Yes
Yes
-
-
Nebraska
Yes
No
Yes
Yes
Yes
-
Yes
c
Nevada
Yes
No
Yes
Yes
Yes
-
-
New Hampshire
Yes
Yes
Yes
Yes
Yes
-
-
New Jersey
Yes
Yes
No
-
Yes
-
No
New Mexico
Yes
Yes
Yes
-
Yes
-
No
New York
Yes
Yes
Yes
-
Yes
-
-
North Carolina
Yes
No
Yes
-
Yes
-
-
North Dakota
Yes
No
Yes
-
Yes
-
-
Ohio
Yes
Yes
c
Yes
-
Yes
-
Yes
Oklahoma
Yes
No
Yes
-
Yes
-
Yes
Oregon
Yes
Yes
Yes
-
Yes
-
No
Pennsylvania
Yes
Yes
Yes
Yes
Yes
-
-
Puerto Rico
Yes
Yes
Yes
-
No
-
No
Rhode Island
Yes
Yes
No
-
No
-
No
South Carolina
Yes
Yes
No
-
Yes
-
Yes
South Dakota
Yes
-
Yes
-
Yes
-
-
Tennessee
Yes
Yes
c
Yes
Yes
Yes
-
Yes
Texas
Yes
Yes
c
Yes
c
-
Yes
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
92
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
U.S. Virgin
Islands
-
-
-
-
-
-
-
Utah
Yes
No
Yes
c
Yes
c
Yes
No
c
-
Vermont
Yes
No
Yes
-
Yes
-
-
Virginia
Yes
Yes
Yes
Yes
Yes
-
No
Washington
Yes
c
No
Yes
-
Yes
-
-
West Virginia
Yes
No
Yes
-
Yes
-
Yes
Wisconsin
Yes
No
Yes
-
No
-
-
Wyoming
Yes
No
Yes
-
Yes
-
Yes
c
Totals for All States
Yes
52
29
40
16
45
0
9
No
0
21
12
0
7
3
10
Unknown
1
3
1
37
1
50
34
Abbreviations: DSM, Diagnostic and Statistical Manual of Mental Disorders; FFS, fee-for-service; MCO, managed
care organization; PDL, Preferred Drug List.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
c
See state information below.
Alabama: The drug is not preferred. Prior authorization is required, and quantity limits apply.
Alaska: The drug is not preferred, and prior authorization, quantity limits, and “medically necessary documentation”
are required.
Arizona: The drug is preferred for pregnant women, and prior authorization is not required if the prescriber indicates
appropriate pregnancy coding.
Arkansas: The formulary identifies the preferred status as “N/A.” The PDL indicates that sublingual is preferred.
The prior authorization criteria and linked memorandum indicate it is preferred with prior authorization criteria. Prior
authorization is required, as are documented counseling and quantity limits.
California: The drug is preferred without limits on the Health and Wellness PDL.
Colorado: The drug is not identified as preferred, and prior authorization and quantity limits are required for FFS
Medicaid.
Connecticut: Buprenorphine sublingual is preferred. The FFY 2017 CMS Drug Utilization Review State
Comparison/Summary Report indicates that, for FFS enrollees, there were some quantity or time limits for
buprenorphine in FFY 2017.
Delaware: Sublingual buprenorphine tablet is preferred with quantity limits.
District of Columbia: The drug is not preferred and requires prior authorization on the FFS PDL. The selected MCO
also requires prior authorization. The FFY 2017 CMS Drug Utilization Review State Comparison/Summary Report
indicates that, for FFS enrollees, there were some quantity or time limits for buprenorphine in FFY 2017.
Florida: Generic sublingual is preferred with prior authorization indicating clinical conditions are met, including
counseling. The prior authorization form asks about failure of other therapies in the prior 12 months, but presumably
this is not a requirement if being administered to a pregnant woman. The FFY 2017 CMS Drug Utilization Review
State Comparison/Summary Report indicates that, for FFS enrollees, there were some quantity or time limits for
buprenorphine in FFY 2017.
Hawaii: It is listed on the FFS formulary. The selected MCO identifies it as preferred without prior authorization or
quantity limits.
Idaho: The drug is nonpreferred; prior authorization is required with multiple requirements, limit of 24 mg/day. Step
therapy is required for all nonpreferred agents, likely not for pregnant women.
Illinois: The buprenorphine hydrochloride is preferred on the FFS PDL, without prior authorization. The FFY 2017
CMS Drug Utilization Review State Comparison/Summary Report indicates that, for FFS enrollees, there were some
quantity or time limits for buprenorphine in FFY 2017.
Indiana: For FFS, generic sublingual is preferred with prior authorization, clinical criteria, counseling, and quantity
limits. It is identified as preferred on a selected MCO formulary.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
93
Iowa: The drug is not preferred; prior authorization, clinical criteria, counseling, and quantity and dose limits apply;
drug is available only for pregnant women.
Kansas: The drug is not on the PDL; it appears that prior authorization, counseling, and quantity limits (24 mg/day)
are required. These requirements are unclear because the buprenorphine requirements incorporate by reference
buprenorphine-naloxone requirements that have been removed, whereas supplemental requirements only applicable
to buprenorphine apply. Authorizations last 3 months.
Kentucky: The drug is not included on the FFS PDL. For multiple MCOs, preferred with prior authorization and, in
some cases, quantity limits and psychosocial treatment are required.
Louisiana: The drug is not preferred for FFS, and prior authorization is required. At least one MCO lists it on its PDL
but imposes quantity limits.
Maine: The drug is not preferred; prior authorization is required; counseling, clinical criteria (i.e., pregnant or proven
allergy), and quantity limits apply. There is a 24-month lifetime limit which, Maine has represented to CMS, offers
unlimited 12-month extensions beyond the 24-month lifetime limitation to persons who meet the state’s medical
necessity criteria for continued methadone treatment (they must be considered successful based on a clinical review
of the ASAM six dimensions placement criteria). Those persons who do not meet the criteria for continued treatment
beyond the 24 months (they are not successful in the program) are referred to other medically necessary services.
Step therapy applies to nonpreferred medications; presumably this would apply to the allergy criteria but not the
pregnancy criteria.
Maryland: The drug is preferred, and there are clinical criteria (pregnant, breastfeeding, or intolerance of naloxone)
after first fill; there also are quantity limits (three tablets/day).
Massachusetts: Prior authorization is required, particularly in certain circumstances when the patient is stable and
has had a recent opioid claim.
Michigan: The state explicitly states there is no lifetime limit on treatment with any buprenorphine drugs for opioid
use disorder treatment.
Minnesota: The drug is preferred for induction. Prior authorization is required.
Mississippi: Buprenorphine is not preferred and requires prior authorization on the FFS PDL. At least one MCO
gives preferred status to buprenorphine sublingual tablets but not to other forms. Quantity limits apply for both FFS
and the MCO.
Missouri: Buprenorphine monotherapy is not preferred, may be prescribed only for pregnant women, and may be
subject to clinical approval. Quantity limits apply.
Montana: The sublingual drug is not preferred for induction or pregnancy only. Counseling and quantity limits apply.
Nebraska: The drug is not preferred, prior authorization is required, and there are quantity limits and clinical criteria
(consent form includes requirement of documented counseling). Nonpreferred required failure on preferred drugs
(unlikely to apply in the case of pregnancy).
New Hampshire: For FFS, the drug is preferred, but prior authorization is required for the entire class of drugs. The
selected MCO lists it as treatment, imposes quantity limits and dose requirements, and requires prior authorization,
clinical criteria, and counseling.
New Jersey: Drug status is preferred on the selected MCO formulary.
New Mexico: Three MCOs identify it as preferred; one requires prior authorization.
New York: The drug is preferred on the FFS PDL with quantity limits. The selected MCO does not prefer it and
requires prior authorization and quantity limits.
North Dakota: The drug is not preferred, prior authorization is required, and quantity and other restrictions apply.
Ohio: The drug is not preferred and requires prior authorization and quantity limits. Step therapy is required in that, if
the patient is not pregnant or breastfeeding, an intolerance of naloxone must be shown. The selected MCO does
identify it as preferred.
Oklahoma: The drug is nonpreferred, prior authorization is required, and dose and quantity limits apply. Other than
for treatment during pregnancy, allergy or adverse reaction to naloxone is required for approval.
Oregon: The FFS PDL does not list buprenorphine, which indicates that prior authorization is required. The FFS
PDL does indicate that quantity limits apply to buprenorphine-containing drugs. The Oregon Health Plan formulary
lists buprenorphine without prior authorization but with quantity limits.
Pennsylvania: Sublingual is preferred with prior authorization, clinical criteria, counseling, and quantity limits. It is to
be used only for induction, during pregnancy, or during breastfeeding.
Puerto Rico: Generic sublingual film is preferred with prior authorization required.
Rhode Island: Generic sublingual tablet is preferred without prior authorization.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
94
South Carolina: The FFS PDL and formulary do not require prior authorization, nor does the selected MCO. The
selected MCO does have quantity limits and imposes step therapy in that, absent pregnancy, there must be a
documented intolerance of naloxone.
South Dakota: The state seemingly does not have a PDL. Quantity limits apply. Prior authorization is required on
the basis of an algorithm that applies to Subutex and Suboxone.
Tennessee: The drug is preferred if the patient is pregnant, prior authorization is required, and there are counseling
and quantity limits. Unless pregnant, inability to take buprenorphine-naloxone must be demonstrated.
Texas: Sublingual buprenorphine is preferred for women who are pregnant and is available without PDL prior
authorization, although clinical authorization is required. The FFY 2017 CMS Drug Utilization Review State
Comparison/Summary Report indicates that, for FFS enrollees, there were some quantity or time limits for
buprenorphine in FFY 2017.
Utah: The drug is nonpreferred with quantity limits, but prior authorization is required only for continuation after 180
days. Continuation also requires documentation of psychosocial support, and continuation after 180 days plus 3
years requires additional justification. Step therapy is required in that it is only available, if not pregnant, with
documented naloxone allergy.
Vermont: The drug is not preferred; prior authorization, clinical criteria related to pregnancy, and quantity limits apply.
Virginia: Buprenorphine sublingual is preferred with prior authorization, counseling, quantity limits and other
restrictions (including pregnancy).
Washington: Buprenorphine monotherapy is covered only for pregnant women who meet DSM-IV criteria for opioid
dependence or DSM-V criteria for moderate or severe opioid disorder; prior authorization and quantity limits apply.
After delivery, the woman must be transitioned to a buprenorphine/naloxone combination medication.
West Virginia: Prior approval is required, and there are limited reasons for authorization. Quantity limits apply.
Absent pregnancy, step therapy is required, in that there must be clinically verified, life-threatening allergy to
naloxone.
Wisconsin: The drug is not preferred, and prior authorization is required.
Wyoming: The drug is not preferred, generic is mandatory, and prior authorization and quantity limits apply. Step
therapy is required in that the patient must have a demonstrated allergy to naloxone. The drug also will be approved
for use during pregnancy.
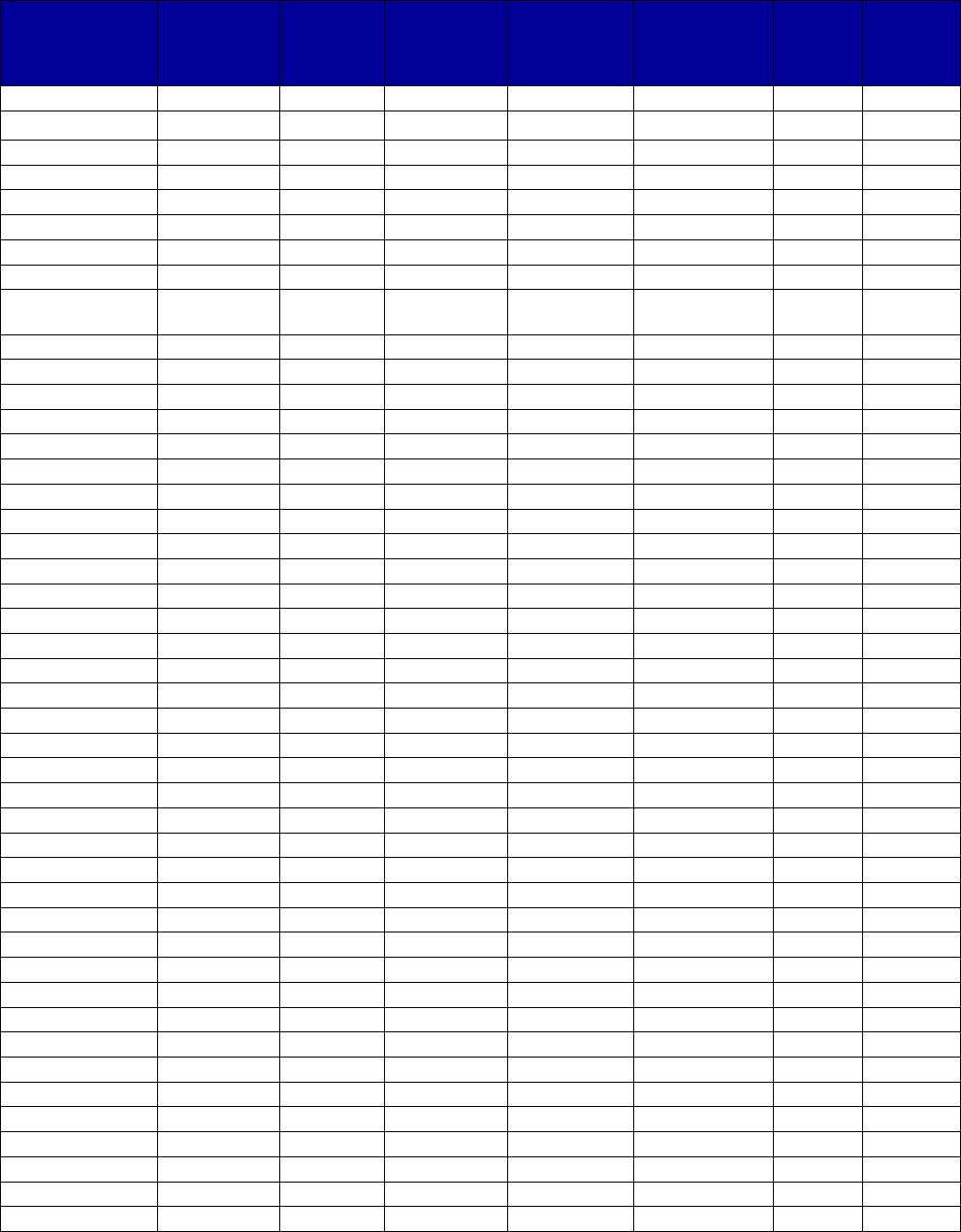
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
95
Table A-8. Medicaid Coverage of Implantable Buprenorphine, by State, 2016–2017
a, b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
No
Yes
-
-
-
-
Alaska Yes No Yes - - - Yes
Arizona
No
-
-
-
-
-
-
Arkansas
Yes
No
-
-
-
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
No
-
-
-
-
-
-
Connecticut
No
-
-
-
-
-
-
Delaware
No
-
-
-
-
-
-
District of
Yes
No
Yes
-
-
-
-
Columbia
Florida
No
-
-
-
-
-
-
Georgia
No
-
-
-
-
-
-
Hawaii
No
-
-
-
-
-
-
Idaho
Yes
No
Yes
-
-
-
Yes
Illinois
Yes
Yes
No
-
-
-
-
Indiana
Yes
-
-
-
-
-
-
Iowa
No
-
-
-
-
-
-
Kansas
Yes
No
Yes
Yes
Yes
-
Yes
Kentucky
Yes
No
Yes
-
Yes
-
-
Louisiana
Yes
No
Yes
-
-
-
-
Maine
Yes
No
Yes
-
-
-
Yes
Maryland
Yes
-
-
-
-
-
-
Massachusetts
Yes
No
Yes
-
-
-
Yes
Michigan
Yes
-
Yes
-
-
-
-
Minnesota
Yes
No
Yes
-
Yes
-
-
Mississippi
Yes
No
Yes
-
-
-
-
Missouri
Yes
No
Yes
No
Yes
-
Yes
Montana
Yes
-
-
-
-
-
-
Nebraska
No
-
-
-
-
-
-
Nevada
No
-
-
-
-
-
-
New Hampshire
Yes
No
Yes
Yes
Yes
-
Yes
New Jersey
Yes
No
Yes
-
No
-
No
New Mexico
Yes
No
Yes
-
No
-
No
New York
Yes
No
Yes
Yes
Yes
Yes
-
North Carolina
Yes
-
Yes
Yes
Yes
-
Yes
North Dakota
Yes
No
Yes
-
Yes
-
-
Ohio
Yes
No
Yes
-
-
-
-
Oklahoma
Yes
No
Yes
-
c
Yes
-
Yes
Oregon
Yes
-
-
-
-
-
-
Pennsylvania
Yes
No
Yes
Yes
Yes
-
-
Puerto Rico
-
-
-
-
-
-
-
Rhode Island
Yes
No
Yes
-
-
-
-
South Carolina
Yes
No
Yes
-
Yes
-
-
South Dakota
No
-
-
-
-
-
-
Tennessee
No
-
-
-
-
-
-
Texas
Yes
-
-
-
-
-
-
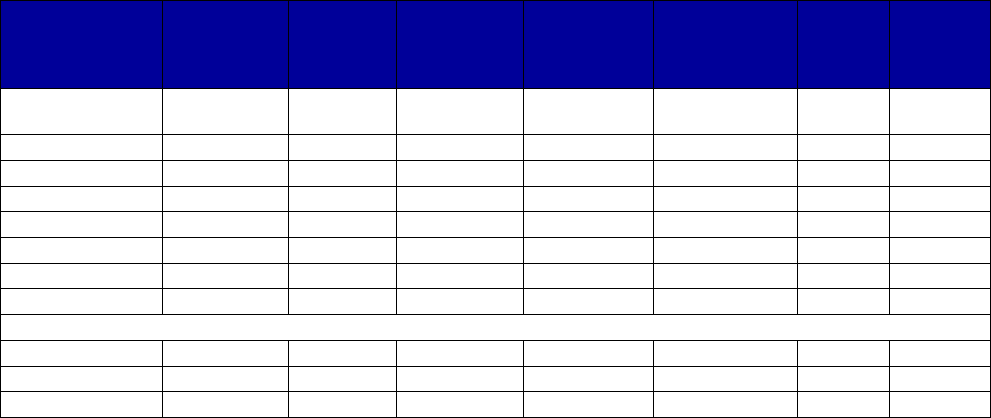
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
96
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
U.S. Virgin
Islands
-
-
-
-
-
-
-
Utah
Yes
-
-
-
-
-
-
Vermont
Yes
No
Yes
-
Yes
-
-
Virginia
Yes
No
No
-
No
-
-
Washington
Yes
No
Yes
-
-
-
-
West Virginia
Yes
-
-
-
Yes
-
-
Wisconsin
No
-
-
-
-
-
-
Wyoming
No
-
-
-
-
-
-
Totals for All States
Yes
37
2
26
5
13
1
9
No
14
26
3
1
4
0
3
Unknown
2
25
24
47
36
52
41
Abbreviations: FFS, fee-for-service; MCO, managed care organization; PDL, Preferred Drug List.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
c
See state information below.
Alaska: At least one failed attempt at treatment with another medication is required to obtain authorization.
Arizona: No evidence of coverage was found.
Arkansas: Probuphine is listed on the PDL as a medical benefit, rather than preferred or nonpreferred.
California: The Health and Wellness PDL identifies it as covered without limits specified.
Colorado: No evidence of coverage was found.
Connecticut: No evidence of coverage was found.
Delaware: No evidence of coverage was found.
District of Columbia: The FFS PDL identifies it as nonpreferred, requiring prior authorization.
Florida: No evidence of coverage was found.
Georgia: No evidence of coverage was found.
Hawaii: No evidence of coverage was found.
Illinois: The drug is preferred without prior authorization.
Indiana: The drug is identified as covered by the code J0570 in provider fee schedules.
Iowa: No evidence of coverage was found.
Kansas: The drug is not on the PDL; prior authorization, counseling, step therapy, and quantity limits are required.
Kentucky: The drug is not on the PDL for any plan examined, although one plan does require prior authorization and
imposes quantity limits.
Louisiana: The FFS PDL identifies Probuphine as requiring prior authorization.
Maine: Probuphine is not preferred and requires prior authorization and failure of preferred treatment. Given the
structure of the PDL, it is unclear what quantity limits, psychosocial counseling requirements, and lifetime limits may
apply specifically to Probuphine.
Massachusetts: Prior authorization requires showing an allergic reaction, contraindication, or inadequate response
to alternatives.
Michigan: The drug is covered as a medical benefit.
Mississippi: The FFS PDL identifies it as covered and not preferred. At least one MCO does not cover it.
Montana: The drug is covered as a medical benefit.
Nebraska: The formulary explicitly does not cover the drug.
Nevada: No evidence of coverage was found.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
97
New Hampshire: The drug is not listed on the FFS PDL, but it is authorized as treatment by the selected MCO, with
prior authorization, quantity limits, counseling, step therapy, and an expectation but not a mandate that treatment not
be longer than 12 months.
New Mexico: One MCO lists it as covered and nonpreferred with prior authorization required.
New Jersey: A selected MCO PDL look-up identifies it as nonpreferred with prior authorization.
New York: The drug is not on the FFS PDL, but it is identified as nonpreferred with limits by the selected MCO.
These include prior authorization, quantity limits, counseling, and step therapy. Use beyond 1 year is considered
investigational and not medically necessary.
North Dakota: The formulary identifies it as covered with prior authorization and quantity limits.
Ohio: The drug is not preferred and requires prior authorization.
Oklahoma: Probuphine is not preferred; prior authorization is required, and there are quantity limits and step therapy
requirements.
Oregon: The drug is listed on the Oregon Health Plan FFS fee schedule.
Pennsylvania: The FFS PDL identifies the drug as nonpreferred.
Puerto Rico: There is no evidence of coverage.
Rhode Island: The FFS PDL identifies it as nonpreferred with prior authorization required.
South Carolina: The FFS and selected MCO formularies identify it as nonpreferred. Prior authorization and quantity
limits are imposed by the FFS formulary.
South Dakota: There is no evidence of coverage.
Tennessee: There is no evidence of coverage.
Texas: The drug is listed on the provider fee schedule.
Utah: The drug is listed on the provider fee schedule.
Virginia: The drug lookup lists it as covered with no limitations identified.
Washington: The PDL identifies it as nonpreferred with prior authorization required.
West Virginia: The drug code list identifies it as reimbursed with quantity limits.
Wisconsin: There is no evidence of coverage.
Wyoming: There is no evidence of coverage.
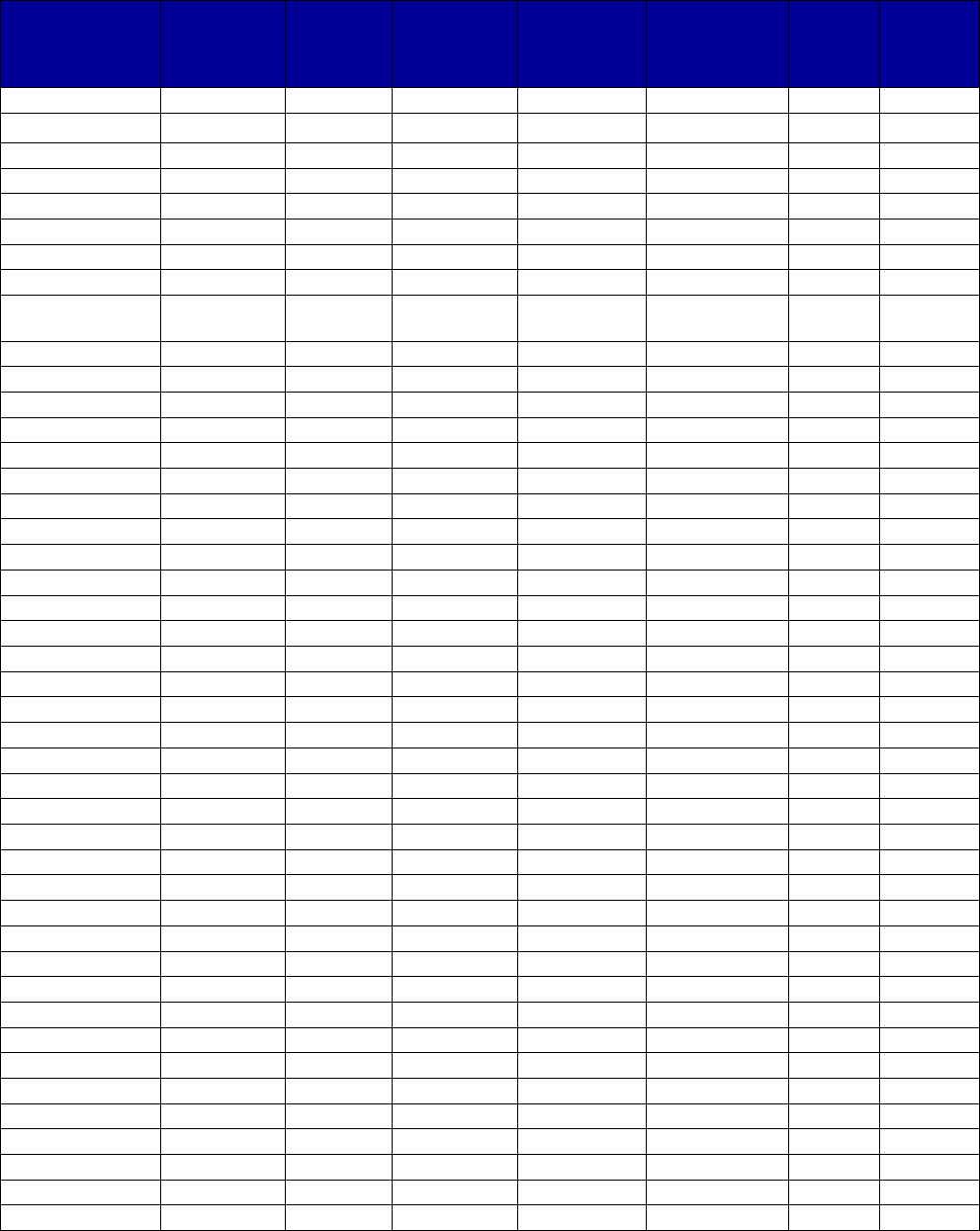
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
98
Table A-9. Medicaid Coverage of Extended-Release Injectable Buprenorphine, by State, 2016–
2017
a,b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
No
No
-
Yes
-
-
Alaska
No
-
-
-
-
-
-
Arizona
No
-
-
-
-
-
-
Arkansas
Yes
No
-
-
-
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
Yes
No
Yes
Yes
Yes
-
-
Connecticut
No
-
-
-
-
-
-
Delaware
Yes
Yes
No
-
-
-
-
District of
Yes No Yes - - - -
Columbia
Florida
No
-
-
-
-
-
-
Georgia
No
-
-
-
-
-
-
Hawaii
No
-
-
-
-
-
-
Idaho
Yes
No
Yes
-
-
-
Yes
Illinois
Yes
Yes
No
-
-
-
-
Indiana
Yes
No
Yes
Yes
Yes
-
-
Iowa
No
-
-
-
-
-
-
Kansas
No
-
-
-
-
-
-
Kentucky
Yes
No
Yes
-
Yes
-
-
Louisiana
No
-
-
-
-
-
-
Maine
Yes
No
Yes
-
-
-
Yes
Maryland
Yes
No
Yes
Yes
Yes
-
No
Massachusetts
Yes
No
Yes
-
-
-
-
Michigan
Yes
No
Yes
Yes
Yes
No
-
Minnesota
Yes
No
Yes
-
Yes
-
-
Mississippi
Yes
No
Yes
-
-
-
No
Missouri
Yes
No
Yes
-
-
-
-
Montana
No
-
-
-
-
-
-
Nebraska
No
-
-
-
-
-
-
Nevada
No
-
-
-
-
-
-
New Hampshire
Yes
No
Yes
Yes
Yes
-
Yes
New Jersey
Yes
-
No
-
Yes
-
No
New Mexico
Yes
-
No
-
Yes
-
No
New York
Yes
Yes
Yes
Yes
Yes
-
-
North Carolina
Yes
Yes
No
-
Yes
-
-
North Dakota
Yes
No
Yes
-
Yes
-
-
Ohio
Yes
Yes
c
Yes
-
Yes
-
-
Oklahoma
No
-
-
-
-
-
-
Oregon
No
-
-
-
-
-
-
Pennsylvania
Yes
No
Yes
Yes
Yes
-
-
Puerto Rico
-
-
-
-
-
-
-
Rhode Island
Yes
No
Yes
-
-
-
-
South Carolina
Yes
Yes
Yes
c
No
No
-
-
South Dakota
No
-
-
-
-
-
-
Tennessee
No
-
-
-
-
-
-
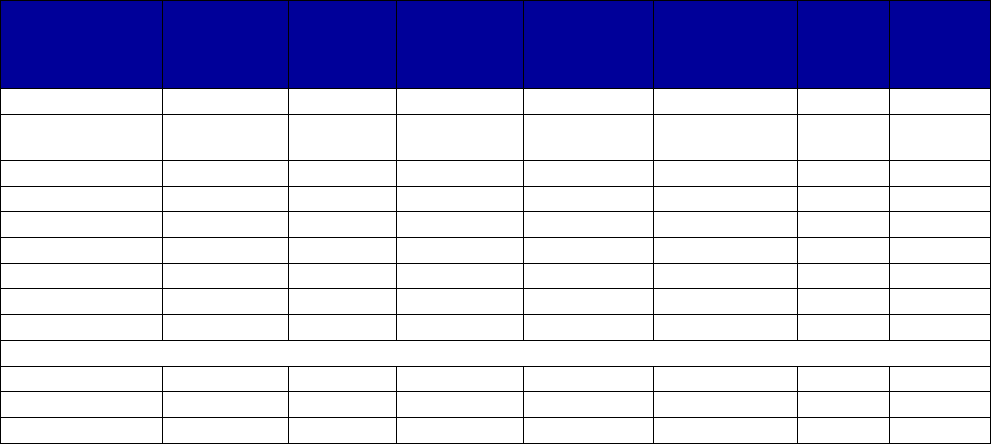
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
99
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Texas
No
-
-
-
-
-
-
U.S. Virgin
Islands
-
-
-
-
-
-
-
Utah
Yes
No
Yes
Yes
-
-
-
Vermont
Yes
No
Yes
-
No
-
-
Virginia
Yes
No
Yes
Yes
Yes
-
-
Washington
Yes
No
Yes
-
-
-
-
West Virginia
Yes
No
Yes
-
Yes
-
-
Wisconsin
Yes
No
Yes
Yes
-
-
-
Wyoming
No
-
-
-
-
-
-
Totals for All States
Yes
33
7
25
10
17
0
3
No
18
24
7
1
3
1
5
Unknown
2
22
21
42
33
52
45
Abbreviations: FFS, fee-for-service; HCPCS, Healthcare Common Procedure Coding System; MAT, medication-
assisted treatment; MCO, managed care organization; PDL, Preferred Drug List.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
c
See state information below.
Alaska: No evidence of coverage was found.
Arizona: No evidence of coverage was found.
Arkansas: Sublocade is listed on the PDL as a medical benefit, rather than preferred or nonpreferred.
California: The Health and Wellness PDL identifies it as covered without limits specified.
Colorado: This drug is approved to be administered only in a long-term care facility or the person’s home by a home
health care professional, if the person is in psychosocial treatment and with quantity limits. Other limits also apply.
Connecticut: No evidence of coverage was found.
Delaware: The drug is listed as preferred on the PDL.
District of Columbia: The FFS PDL identifies it as nonpreferred, requiring prior authorization.
Florida: No evidence of coverage was found.
Georgia: No evidence of coverage was found.
Hawaii: No evidence of coverage was found.
Illinois: The drug is preferred without prior authorization.
Indiana: The drug is nonpreferred but reimbursed with limits.
Iowa: No evidence of coverage was found.
Kansas: We did not find the drug listed on the PDLs checked nor the HCPCS code on the professional fee schedule.
Kentucky: This drug is identified as nonpreferred on one managed care formulary, with prior authorization and
quantity limits required.
Louisiana: We did not find the drug listed on the PDLs checked nor the HCPCS code on the professional fee
schedule.
Maine: Sublocade is not preferred and requires prior authorization and failure of preferred treatment. Given the
structure of the PDL, it is unclear what quantity limits, psychosocial counseling requirements, and lifetime limits may
apply specifically to the drug.
Michigan: The state explicitly states there is no lifetime limit on MAT treatment with buprenorphine drugs.
Montana: There is no evidence of coverage in Montana.
Nebraska: The formulary explicitly does not cover the drug.
Nevada: No evidence of coverage was found.
New Hampshire: The drug is not on the FFS PDL or one MCO PDL. It is listed with limits on a second MCO
formulary.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
100
New Jersey: A selected MCO PDL look-up identifies it as a medical benefit with quantity limits but no prior
authorization.
New Mexico: Only one MCO identifies it as covered, and that is as a medical benefit without prior authorization.
New York: Preferred on FFS PDL. The selected MCO considers it nonpreferred and imposes limits.
North Dakota: The formulary identifies it as covered with prior authorization and quantity limits.
Ohio: The drug is preferred within the FFS plan with prior authorization and quantity limits. The selected MCO does
not list it on the PDL.
Oklahoma: There is no evidence of coverage.
Oregon: There is no evidence of coverage.
Pennsylvania: This drug is covered as nonpreferred with prior authorization, counseling, and quantity limits.
Puerto Rico: There is no evidence of coverage.
Rhode Island: The FFS PDL identifies the drug as not preferred and requires prior authorization.
South Carolina: The FFS PDL identifies the drug as preferred without prior authorization. The FFS formulary
indicates prior authorization is required. The drug is treated as a nonpreferred medical benefit on the selected MCO
formulary.
South Dakota: There is no evidence of coverage.
Tennessee: There is no evidence of coverage.
Texas: The physician fee schedule look-up checked indicates it is not payable.
Utah: The drug is nonpreferred on the FFS PDL and requires clinical prior authorization with documented
psychosocial services.
Virginia: The drug is listed on the PDL as not preferred and requires prior authorization, quantity limits, and
participation in counseling.
Washington: The PDL identifies the drug as not preferred and requires prior authorization.
West Virginia: The PDL identifies the drug as not preferred and requires prior authorization and quantity limits.
Wisconsin: The drug is nonpreferred and requires prior authorization and participation in counseling.
Wyoming: There is no evidence of coverage.
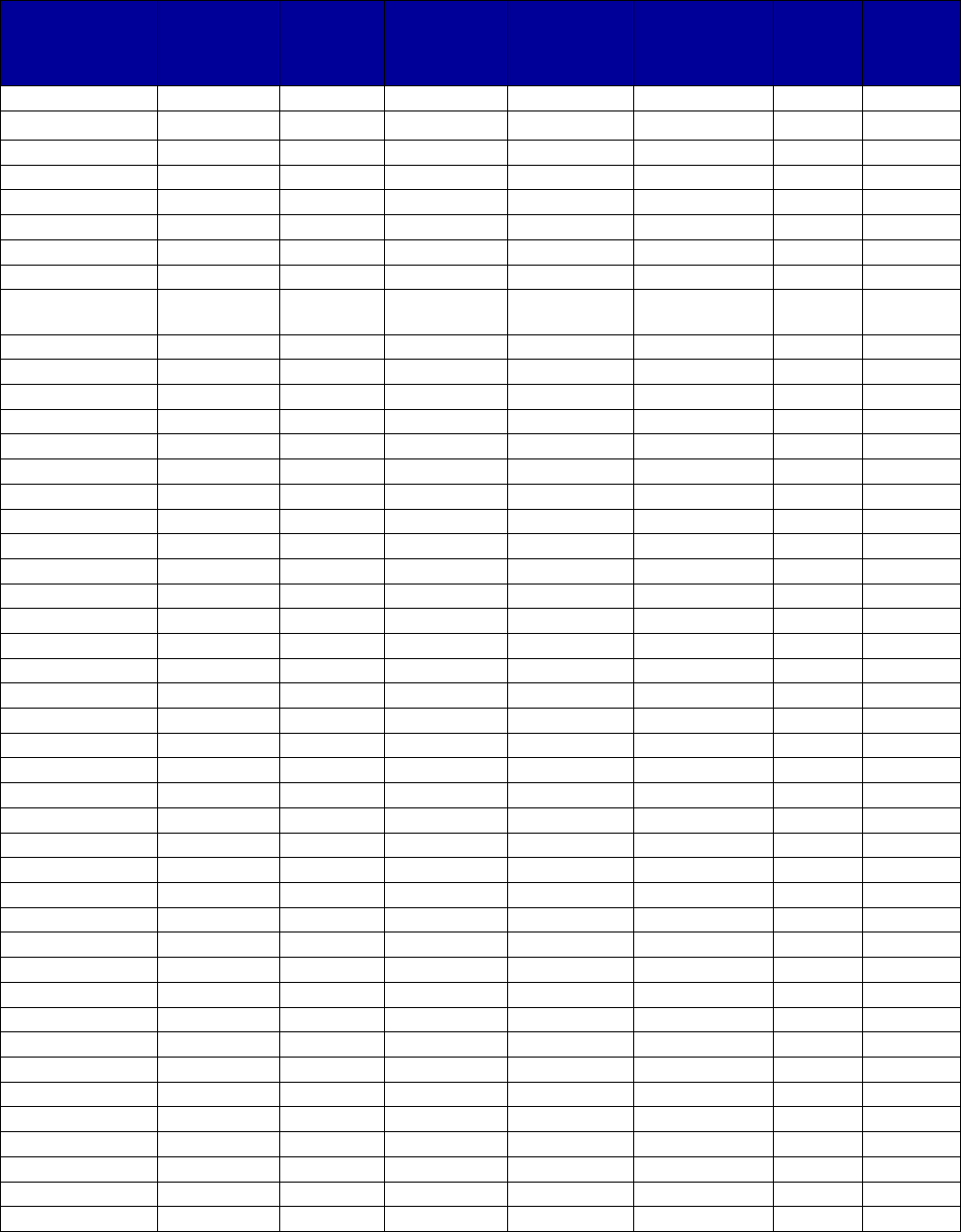
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
101
Table A-10. Medicaid Coverage of Buprenorphine-Naloxone, by State, 2016–2017
a,b
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
Alabama
Yes
Yes
Yes
-
Yes
-
-
Alaska Yes Yes Yes - Yes - -
Arizona
Yes
Yes
No
-
No
-
-
Arkansas
Yes
Yes
Yes
Yes
Yes
-
-
California
Yes
Yes
No
-
No
-
No
Colorado
Yes
Yes
Yes
-
Yes
-
-
Connecticut
Yes
Yes
No
-
Yes
-
-
Delaware
Yes
Yes
No
-
Yes
-
No
District of
Yes
Yes
No
-
Yes
-
-
Columbia
Florida
Yes
Yes
Yes
Yes
Yes
-
Yes
Georgia
Yes
Yes
No
-
Yes
-
-
Hawaii
Yes
Yes
No
-
No
-
-
Idaho
Yes
Yes
Yes
-
Yes
-
No
Illinois
Yes
Yes
No
-
Yes
-
-
Indiana
Yes
Yes
Yes
Yes
Yes
-
-
Iowa
Yes
Yes
Yes
Yes
Yes
-
-
Kansas
Yes
No
No
-
Yes
-
-
Kentucky
Yes
Yes
Yes
Yes
Yes
-
-
Louisiana
Yes
Yes
No
-
Yes
-
-
Maine
Yes
Yes
Yes
Yes
Yes
No
c
-
Maryland
Yes
Yes
No
-
Yes
-
-
Massachusetts
Yes
Yes
Yes
-
Yes
-
Yes
Michigan
Yes
Yes
Yes
Yes
Yes
No
No
Minnesota
Yes
Yes
Yes
-
Yes
-
-
Mississippi
Yes
Yes
No
-
Yes
-
No
Missouri
Yes
Yes
Yes
-
Yes
-
No
Montana
Yes
Yes
Yes
Yes
Yes
-
-
Nebraska
Yes
Yes
No
Yes
Yes
-
No
Nevada
Yes
Yes
Yes
Yes
Yes
-
-
New Hampshire
Yes
Yes
No
Yes
Yes
-
-
New Jersey
Yes
Yes
Yes
-
Yes
-
No
New Mexico
Yes
Yes
Yes
-
Yes
-
No
New York
Yes
Yes
Yes
-
Yes
-
-
North Carolina
Yes
Yes
No
-
Yes
-
-
North Dakota
Yes
Yes
Yes
-
Yes
-
-
Ohio
Yes
Yes
Yes
Yes
Yes
-
-
Oklahoma
Yes
Yes
Yes
c
-
Yes
-
-
Oregon
Yes
Yes
No
-
Yes
-
No
Pennsylvania
Yes
Yes
Yes
Yes
Yes
-
-
Puerto Rico
Yes
Yes
Yes
-
No
-
No
Rhode Island
Yes
Yes
No
-
No
-
No
South Carolina
Yes
Yes
No
-
Yes
-
No
South Dakota
Yes
Yes
Yes
-
Yes
-
-
Tennessee
Yes
Yes
Yes
Yes
Yes
-
-
Texas
Yes
Yes
Yes
c
-
Yes
-
-

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
102
State
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity limits
or maximum
daily doses?
Are
there
lifetime
limits?
Is step
therapy
used?
U.S. Virgin
Islands
-
-
-
-
-
-
-
Utah
Yes
Yes
No
c
Yes
c
Yes
No
c
-
Vermont
Yes
Yes
No
-
Yes
-
-
Virginia
Yes
Yes
Yes
Yes
Yes
-
No
Washington
Yes
Yes
No
c
-
Yes
No
c
-
West Virginia
Yes
Yes
Yes
-
Yes
-
-
Wisconsin
Yes
Yes
Yes
-
No
-
-
Wyoming
Yes
Yes
Yes
-
Yes
-
No
Totals for All States
Yes
52
51
31
16
46
0
2
No
0
1
21
0
6
4
15
Unknown
1
1
1
37
1
49
36
Abbreviations: DSM, Diagnostic and Statistical Manual of Mental Disorders; FFS, fee-for-service; MAT, medication-
assisted treatment; MCO, managed care organization; PDL, Preferred Drug List; SL, sublingual.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
c
See state information below.
Alabama: Suboxone is preferred. Prior authorization is required, and quantity limits apply.
Alaska: Suboxone film and buprenorphine-naloxone SL are preferred; others are not. Prior authorization, quantity
limits, and “medically necessary documentation” are required.
Arizona: Only Suboxone film is preferred.
Arkansas: Suboxone film is preferred with prior authorization, quantity limits, and counseling required. Other
versions are not preferred.
California: The Health and Wellness PDL identifies it as covered without limits being identified.
Colorado: Suboxone is preferred, prior authorization is required, there are quantity limits.
Connecticut: Suboxone film is preferred. The FFY 2017 CMS Drug Utilization Review State Comparison/Summary
Report indicates that, for FFS enrollees, there were some quantity or time limits for buprenorphine-naloxone
formulations in FFY 2017.
Delaware: Suboxone and Zubsolv are preferred, but there are quantity limits.
District of Columbia: Suboxone film is preferred and other formulations are nonpreferred. Prior authorization is
required for nonpreferred drugs. The selected MCO does not require prior authorization unless the dose is above the
established quantity limit. The FFY 2017 CMS Drug Utilization Review State Comparison/Summary Report indicates
that, for FFS enrollees, there were some quantity or time limits for buprenorphine-naloxone formulations in FFY 2017.
Florida: Suboxone film is preferred with prior authorization required indicating clinical criteria are met, including
participation in counseling and, apparently, failure of some previous treatment in the past 12 months. The FFY 2017
CMS Drug Utilization Review State Comparison/Summary Report indicates that, for FFS enrollees, there were some
quantity or time limits for buprenorphine-naloxone formulations in FFY 2017.
Georgia: Suboxone is preferred; others are not. Prior authorization is required for nonpreferred versions. Quantity
limits apply.
Hawaii: The FFS formulary does not include it, but the selected MCO identifies it as preferred without prior
authorization or quantity limits.
Idaho: Suboxone film is preferred, and other formulations are nonpreferred. Prior authorization and quantity limits
are required for all versions. Step therapy is required for all nonpreferred agents, and other requirements exist.
Illinois: The generic Suboxone, Bunavail, and Zubsolv are all preferred on the FFS PDL. The FFY 2017 CMS Drug
Utilization Review State Comparison/Summary Report indicates that, for FFS enrollees, there were some quantity or
time limits for buprenorphine-naloxone formulations in FFY 2017.
Indiana: For FFS, Suboxone and the generic are preferred; the remainder are not. All require prior authorization and
have clinical criteria, counseling, and quantity limits.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
103
Iowa: Suboxone is preferred; all others are not preferred. All require prior authorization, clinical criteria, counseling,
and quantity and dose limits. Step therapy is required for nonpreferred drugs.
Kansas: The FFY 2017 CMS Drug Utilization Review State Comparison/Summary Report indicates that, for FFS
enrollees, there were some quantity or time limits for buprenorphine-naloxone formulations in FFY 2017.
Kentucky: The drug is not included on the FFS PDL. Multiple MCOs may have a drug preference (which depends
on the MCO). All require prior authorization and, in some cases, quantity limits that vary by formulation. One
requires failure first with generic to obtain brand.
Louisiana: For FFS, buprenorphine-naloxone and Suboxone film are preferred; others are not preferred and require
prior authorization. At least one MCO imposes prior authorization and quantity limits.
Maine: Suboxone film is preferred; the remainder are not. For all versions, prior authorization is required, and there
are counseling, clinical criteria, and quantity limits. Preferred must be tried before nonpreferred. For Bunavail and
Zubsolv, authorization may be granted after 24 months if certain criteria are met. There is a 24-month lifetime limit in
both the PDL and in the Maine statutes which Maine has represented to CMS offers unlimited 12-month extensions
beyond the 24-month lifetime limitation to persons who meet the state’s medical necessity criteria for continued
methadone treatment (they must be considered successful based on a clinical review of the ASAM six dimensions
placement criteria). Those persons who do not meet the criteria for continued treatment beyond the 24 months (they
are not successful in the program) are referred to other medically necessary services.
Maryland: Suboxone film, Bunavail, and Zubsolv are preferred; Suboxone that is not film is not preferred and
requires prior authorization. All have quantity limits that vary by dose and formulation.
Massachusetts: Suboxone film is preferred but with prior authorization, and the type of authorization documentation
varies by dose, route, and whether the person also is prescribed an opioid.
Michigan: The state explicitly states that there is no lifetime limit on MAT treatment with buprenorphine products.
Minnesota: Suboxone film is preferred; other formulations are not. All require prior authorization. There is a quantity
limit of 24 mg/day for all but Zubsolv, which has different limits based on dose. Zubsolv requires step therapy.
Mississippi: Suboxone film is preferred in the FFS program. Among nonpreferred drugs, Bunavail is preferred over
others including generic, although Bunavail is not indicated for induction and has certain step therapy requirements.
All require prior authorization, and quantity limits and other restrictions apply. At least one MCO prefers SL tablets
and does not require prior authorization for that formulation.
Missouri: Suboxone film is preferred, but it is likely that prior authorization continues to be required. Quantity limits
apply and vary depending on route, dose, and stage of treatment. Step therapy is required only for nonpreferred
formulations.
Montana: Suboxone film is preferred with prior authorization required; all others are not preferred. There are
counseling and quantity limits, and annual updates are required.
Nebraska: Suboxone film is preferred, the remainder are not preferred. Prior authorization is required for all versions
except the preferred; quantity limits vary by formulation. There are clinical criteria; the consent form includes a
requirement of documented counseling. Nonpreferred requires failure on preferred drugs.
Nevada: Bunavail, Suboxone, and Zubsolv are preferred, with prior authorization, quantity limits, and counseling
required.
New Hampshire: For FFS, Suboxone is preferred and the remaining drugs are not preferred; prior authorization is
required for all. One selected MCO prefers Suboxone film and does not require prior authorization for lower doses of
the preferred drug, but it does impose prior authorization and failure with the preferred drug for nonpreferred
approval. It also imposes quantity limits and dose requirements, clinical criteria, and counseling.
New Jersey: On a selected MCO PDL, the status was preferred with quantity limits and prior authorization.
New Mexico: The drug is preferred by at least two MCOs, although one requires prior authorization.
New York: For FFS and the selected MCO, Suboxone film is preferred; the remaining drugs are not preferred and
require prior authorization. Quantity limits apply.
North Carolina: Suboxone film is preferred and the remaining drugs are not. Trial and failure of two preferred
agents are required before authorization for nonpreferred is allowed. Prior authorization is not required for Suboxone
film.
North Dakota: Zubsolv is preferred with prior authorization and quantity limits. Other formulations are not preferred.
Ohio: For FFS, Suboxone film and Zubsolv tablets are preferred with prior authorization, counseling, and quantity
limits. The selected MCO identifies generic SL tablets and the generic film as preferred with prior authorization
required.
Oklahoma: Suboxone film is preferred and the remaining drugs are nonpreferred. Prior authorization is required,
although it is less than clear for Suboxone; dose and quantity limits apply depending on the formulation. Step therapy
is required only for nonpreferred formulations.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
104
Oregon: The FFS formulary lists suboxone film, Zubsolv, and the generic SL tablet as covered with quantity limits.
The Oregon Health Plan formulary lists the generic without prior authorization but with quantity limits. Prior
authorization would be required for the brands not listed on the FFS PDL or Oregon Health Plan formulary.
Pennsylvania: Suboxone film is preferred; all others are nonpreferred. All drugs require prior authorization, clinical
criteria, counseling, and quantity limits.
Puerto Rico: Suboxone film is preferred with prior authorization required.
Rhode Island: Suboxone film is preferred without prior authorization on the FFS PDL. A selected MCO prefers the
SL generic tablet.
South Carolina: The FFS PDL and formulary prefer Suboxone and the generic SL tablet without limits. The selected
MCO identifies Suboxone film and generic buprenorphine/naloxone as preferred but includes quantity limits.
South Dakota: The state seemingly does not have a PDL. Quantity limits and prior authorization apply. The FFY
2017 CMS Drug Utilization Review State Comparison/Summary Report indicates that, for FFS enrollees, there was at
least one preferred buprenorphine-naloxone formulation in FFY 2017.
Tennessee: Only Bunavail is preferred; the remaining drugs are not preferred. All require prior authorization,
counseling, and quantity limits. Step therapy is required for nonpreferred formulations only.
Texas: Only Suboxone film is preferred. All require clinical authorization, and all but Suboxone require PDL prior
authorization. The FFY 2017 CMS Drug Utilization Review State Comparison/Summary Report indicates that, for FFS
enrollees, there were some quantity or time limits for buprenorphine-naloxone formulations in FFY 2017.
Utah: Suboxone is preferred; Bunavail, generic, and Zubsolv are not preferred. The drug has quantity limits, but prior
authorization is required only for continuation after 180 days. Continuation also requires documentation of
psychosocial support, and continuation after 180 days plus 3 years requires additional justification.
Vermont: Suboxone film is preferred; the remaining drugs are not preferred. Prior authorization (only for
nonpreferred), clinical criteria, and quantity limits apply.
Virginia: Suboxone film is preferred with prior authorization. There are requirements for counseling and quantity
limits, and there are other restrictions.
Washington: Buprenorphine/naloxone is preferred without limits up to a dose of 24 mg/day for individuals aged 16
years and older who meet DSM-IV criteria for opioid dependence or DSM-V criteria for moderate or severe opioid
disorder. Otherwise, prior authorization is required. Additionally, prescribers are required to follow several
prescribing guidelines “voluntarily”; failure to do so as a prescriber community may result in reinstitution of prior
authorization requirements. Included in the voluntary guidelines are participation in more comprehensive treatment,
induction limits, and other restrictions. Washington state MAT guidelines expressly state that there is no lifetime limit
for buprenorphine.
West Virginia: Suboxone film is preferred but still has clinical and quantity limits. All others are nonpreferred.
Wisconsin: Suboxone film and Zubsolv are preferred; remaining drugs are not preferred. Prior authorization is
required.
Wyoming: Suboxone film is preferred; clinical criteria, prior authorization, and quantity limits apply. Step therapy is
required only for nonpreferred forms.
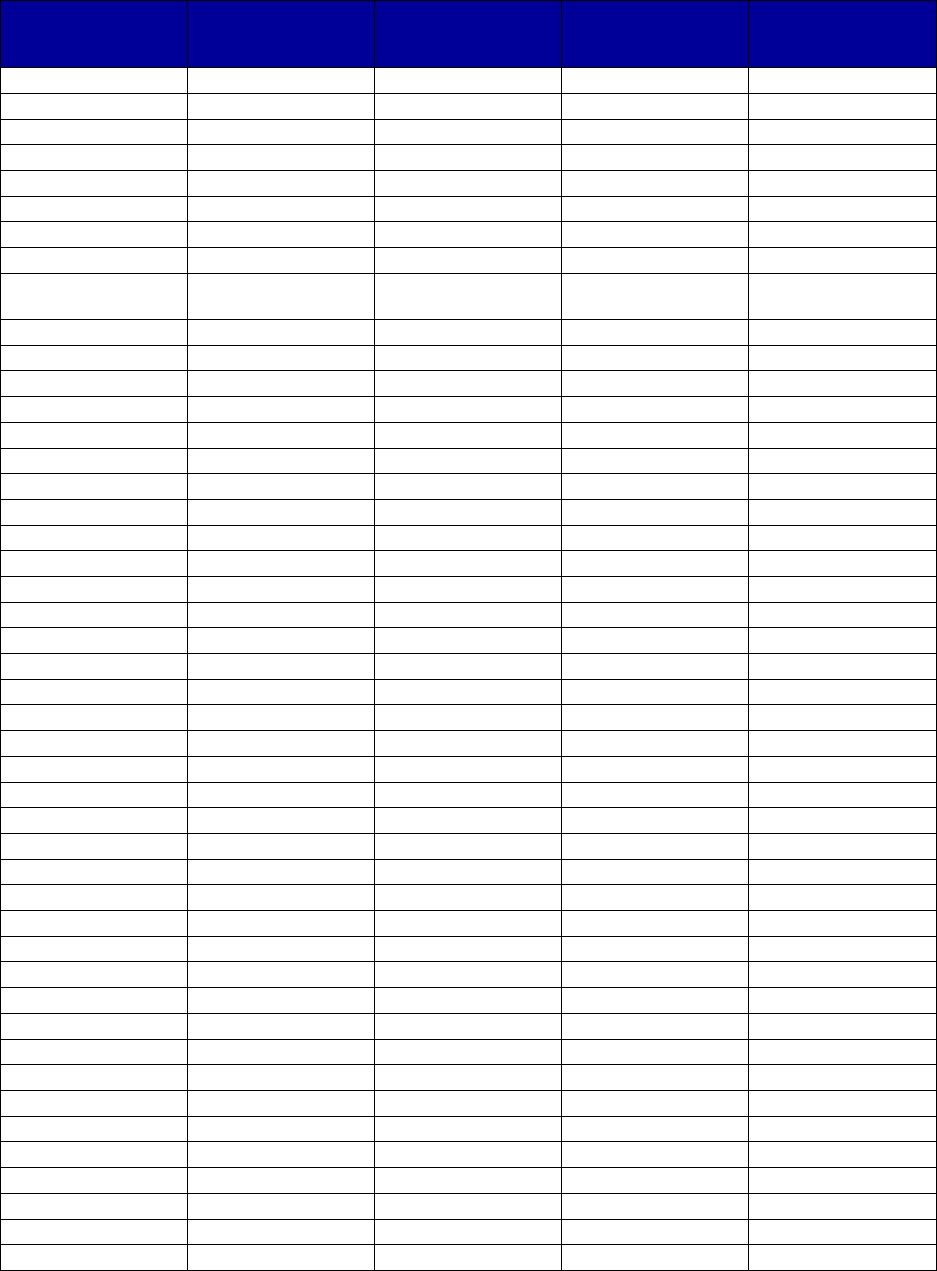
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
105
Table A-11. Medicaid Coverage of Methadone as MAT, by State, 2016–2017
a,b
State
Is this drug
covered by
Medicaid?
Is prior
authorization
required?
c
Are there quantity
limits or maximum
daily doses?
Are there lifetime
limits?
Alabama
Yes
-
-
-
Alaska
Yes
c
-
-
-
Arizona
Yes
-
-
-
Arkansas
No
-
-
-
California
Yes
-
-
-
Colorado
Yes
-
-
-
Connecticut
Yes
Yes
-
-
Delaware
Yes
-
-
-
District of
Yes
-
-
-
Columbia
Florida
Yes
-
-
-
Georgia
Yes
-
-
-
Hawaii
Yes
-
-
-
Idaho
No
-
-
-
Illinois
Yes
-
-
-
Indiana
Yes
-
-
-
Iowa
Yes
-
-
-
Kansas
Yes
c
-
-
-
Kentucky
No
-
-
-
Louisiana
No
-
-
-
Maine
Yes
Yes
Yes
Yes
c
Maryland
Yes
-
-
-
Massachusetts
Yes
-
-
-
Michigan
Yes
-
-
-
Minnesota
Yes
-
-
-
Mississippi
Yes
c
-
-
-
Missouri
Yes
c
-
-
-
Montana
Yes
c
-
-
-
Nebraska
No
-
-
-
Nevada
Yes
-
-
-
New Hampshire
Yes
-
-
-
New Jersey
Yes
No
-
-
New Mexico
Yes
-
-
-
New York
Yes
-
-
-
North Carolina
Yes
Yes
-
-
North Dakota
No
-
-
-
Ohio
Yes
-
-
-
Oklahoma
Yes
c
-
-
-
Oregon
Yes
-
-
-
Pennsylvania
Yes
-
-
-
Puerto Rico
-
-
-
-
Rhode Island
Yes
No
-
-
South Carolina
No
-
-
-
South Dakota
Yes
c
-
-
-
Tennessee
No
-
-
-
Texas
Yes
-
-
-
U.S. Virgin Islands
-
-
-
-
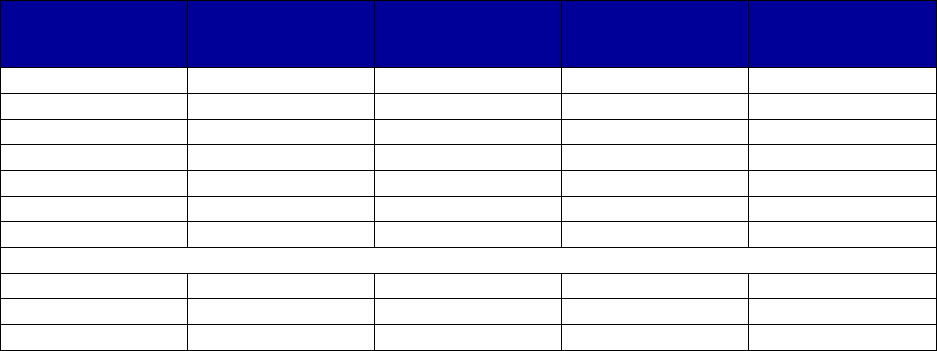
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
106
State
Is this drug
covered by
Medicaid?
Is prior
authorization
required?
c
Are there quantity
limits or maximum
daily doses?
Are there lifetime
limits?
Utah
Yes
-
-
-
Vermont
Yes
-
-
-
Virginia
Yes
-
-
-
Washington
Yes
-
-
-
West Virginia
Yes
No
-
-
Wisconsin
Yes
-
-
-
Wyoming
No
-
-
-
Totals for All States
Yes
42
3
1
1
No
9
3
0
0
Unknown
2
47
52
52
Abbreviation: MAT, medication-assisted treatment.
a
Because of the unique regulatory structure around methadone as MAT for substance use disorders (in contrast to
use for treatment of pain), some requirements such as counseling, step therapy for those younger than 18 years old,
and daily dosing requirements are uniformly part of the federal regulations governing dispensing of methadone in an
opioid treatment program. For these reasons, only certain unique state-specific limits are addressed in this table. In
general, preferred status is not a relevant inquiry for methadone treatment of opioid use disorder.
b
Materials reviewed were those available in the second and third quarters of 2018.
c
Additional requirements for prior authorization may exist as imposed by specific state Medicaid managed care
organizations.
Alabama: The FY2017 Kaiser Family Foundation Medicaid Budget Survey summary indicates that Alabama reports
not reimbursing methadone for purposes of MAT. A 2017 Alabama Medicaid document indicates that code H0020 is
covered. We are counting it as covered.
Alaska: We find no evidence that methadone is covered for purposes of MAT in any Medicaid documents. The
Kaiser Family Foundation State Health Facts based on the FY2017 Medicaid Budget Survey summary indicate that
Alaska reports reimbursing methadone for purposes of MAT. We are counting it as covered.
Connecticut: Prior authorization is required for code H0020.
Kansas: We find no evidence that methadone is covered for purposes of MAT in any Medicaid documents. The
Kaiser Family Foundation State Health Facts based on the FY2017 Medicaid Budget Survey summary indicate that
Kansas reports reimbursing methadone for purposes of MAT. We are counting it as covered.
Maine: Treatment with methadone is limited to 24 months absent prior authorization.
Mississippi: We find no evidence that methadone is covered for purposes of MAT in any Medicaid documents. The
Kaiser Family Foundation State Health Facts based on the FY2017 Medicaid Budget Survey summary indicate that
Mississippi reports reimbursing methadone for purposes of MAT. We are counting it as covered.
Missouri: The Kaiser Family Foundation State Health Facts based on the FY2017 Medicaid Budget Survey summary
indicate that Missouri reports reimbursing methadone for purposes of MAT and the state has advised CMS that it has
multiple sites furnishing methadone, with Medicaid as a payer.
Montana: We find no evidence that methadone is covered for purposes of MAT in any Medicaid documents. The
Kaiser Family Foundation State Health Facts based on the FY2017 Medicaid Budget Survey summary indicate that
Montana reports reimbursing methadone for purposes of MAT. We are counting it as covered.
North Carolina: Initial authorization shall not exceed 60 days. Reauthorization shall not exceed 180 days. All
utilization review activity shall be documented in the Provider’s Service Plan.
Oklahoma: We find no evidence that methadone is covered for purposes of MAT in any Medicaid documents. The
Kaiser Family Foundation State Health Facts based on the FY2017 Medicaid Budget Survey summary indicate that
Oklahoma reports reimbursing methadone for purposes of MAT. We are counting it as covered.
Rhode Island: Prior authorization is explicitly not required.
South Dakota: We find no evidence that methadone is covered for purposes of MAT in any Medicaid documents.
The Kaiser Family Foundation State Health Facts based on the FY2017 Medicaid Budget Survey summary indicate
that South Dakota reports reimbursing methadone for purposes of MAT. We are counting it as covered.
Virginia: Section 1115 waiver indicates that service authorization is required.
West Virginia: Prior authorization is explicitly not required.
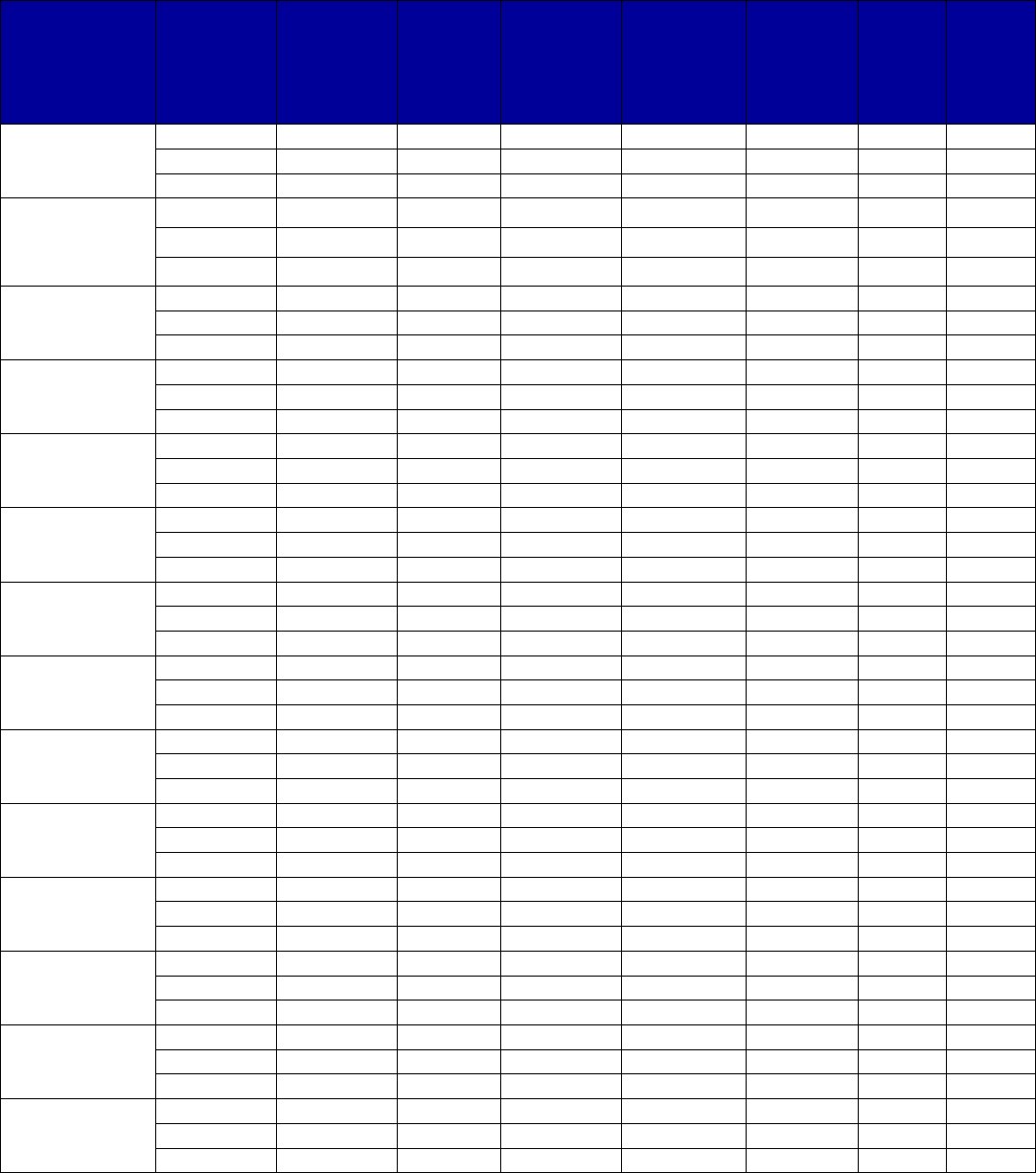
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
107
Table A-12. Medicaid Coverage of Naloxone, by State, 2016–2017
a,b
State Formulation
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity
limits or
maximum
daily
doses?
Are
there
life-
time
limits?
Is step
therapy
used?
Alabama
Naloxone
Yes
No
No
-
Yes
-
-
Narcan
Yes
No
No
-
Yes
-
-
Evzio
No
-
-
-
-
-
-
Alaska
Naloxone
Yes
-
-
-
-
-
-
Narcan
Yes
-
-
-
-
-
-
Evzio Yes No Yes - Yes - -
Arizona
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
No
-
-
-
-
-
-
Arkansas
Naloxone
Yes
No
No
-
-
-
-
Narcan
Yes
No
No
-
Yes
-
-
Evzio
Yes
No
Yes
-
-
-
-
California
Naloxone
Yes
Yes
No
-
No
-
No
Narcan
Yes
Yes
No
-
No
-
No
Evzio
No
-
-
-
-
-
-
Colorado
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
Connecticut
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
Delaware
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
Yes
No
Yes
-
-
-
-
District of
Columbia
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
Yes
No
Yes
-
-
-
-
Florida
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
Georgia
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
Yes
-
No
-
-
Evzio
Yes
No
Yes
-
Yes
-
-
Hawaii
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
No
-
-
Yes
-
-
Evzio
No
-
-
-
-
-
-
Idaho
Naloxone
Yes
Yes
Yes
-
-
-
-
Narcan
Yes
Yes
Yes
-
-
-
-
Evzio
No
-
-
-
-
-
-
Illinois
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
108
State Formulation
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity
limits or
maximum
daily
doses?
Are
there
life-
time
limits?
Is step
therapy
used?
Indiana
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
Yes
No
Yes
-
-
-
-
Iowa
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
Kansas
Naloxone
Yes
No
No
-
-
-
-
Narcan
Yes
No
No
-
-
-
-
Evzio
No
No
-
-
-
-
-
Kentucky
Naloxone
Yes
Yes
Yes
-
Yes
-
-
Narcan
Yes
Yes
Yes
-
Yes
-
-
Evzio
Yes
No
Yes
-
Yes
-
-
Louisiana
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
Yes
No
Yes
-
-
-
-
Maine
Naloxone
Yes
No
Yes
-
-
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
Yes
No
Yes
-
-
-
-
Maryland
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
Yes
No
Yes
-
No
-
-
Massachusetts
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
Michigan
Naloxone
Yes
Yes
No
-
Yes
-
No
Narcan
Yes
Yes
No
-
Yes
-
No
Evzio
No
-
-
-
-
-
-
Minnesota
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
No
-
-
-
-
-
-
Mississippi
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
Yes
No
No
-
-
-
-
Missouri
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
Montana
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
No
-
-
-
-
-
-
Nebraska
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
Nevada
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
Yes
Yes
No
-
Yes
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
109
State Formulation
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity
limits or
maximum
daily
doses?
Are
there
life-
time
limits?
Is step
therapy
used?
New Hampshire
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
Yes
No
Yes
Yes
No
-
-
New Jersey
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
Yes
Yes
No
-
-
-
-
New Mexico
Naloxone
Yes
Yes
No
-
No
-
No
Narcan
Yes
Yes
Yes
-
Yes
-
-
Evzio
Yes
No
Yes
-
Yes
-
No
New York
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
Yes
No
Yes
-
Yes
-
Yes
North Carolina
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
North Dakota
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
c
Yes
Yes
-
-
Evzio
Yes
No
Yes
Yes
Yes
-
-
Ohio
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
No
-
-
-
-
-
-
Oklahoma
Naloxone
Yes
-
-
-
-
-
-
Narcan
Yes
-
-
-
-
-
-
Evzio
No
-
-
-
-
-
-
Oregon
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
Yes
-
Yes
-
-
Evzio
No
-
-
-
-
-
-
Pennsylvania
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
Yes
No
Yes
-
No
-
-
Puerto Rico
Naloxone
-
-
-
-
-
-
-
Narcan
-
-
-
-
-
-
-
Evzio
-
-
-
-
-
-
-
Rhode Island
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
No
-
-
-
-
-
-
South Carolina
Naloxone
Yes
Yes
No
-
Yes
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
Yes
No
Yes
-
Yes
-
Yes
South Dakota
Naloxone
Yes
-
No
-
-
-
-
Narcan
Yes
-
No
-
-
-
-
Evzio
Yes
No
Yes
-
-
-
-
Tennessee
Naloxone
Yes
No
-
-
-
-
-
Narcan
Yes
Yes
Yes
-
Yes
-
-
Evzio
No
-
-
-
-
-
-
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
110
State Formulation
Is this drug
covered by
Medicaid?
Does this
drug have
preferred
status?
Is prior
authoriza-
tion
required?
Is psycho-
social
treatment
required?
Are there
quantity
limits or
maximum
daily
doses?
Are
there
life-
time
limits?
Is step
therapy
used?
Texas
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
No
-
-
-
-
-
-
U.S. Virgin
Islands
- - - - - - - -
Utah
Naloxone
Yes
-
-
-
-
-
-
Narcan
Yes
-
-
-
-
-
-
Evzio
No
-
-
-
-
-
-
Vermont
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
Yes
-
-
Evzio
No
-
-
-
-
-
-
Virginia
Naloxone
Yes
Yes
No
-
-
-
-
Narcan
Yes
Yes
No
-
-
-
-
Evzio
Yes
No
Yes
-
-
-
-
Washington
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
No
-
-
-
-
-
-
West Virginia
Naloxone
Yes
Yes
No
-
Yes
c
-
-
Narcan
Yes
Yes
No
-
No
c
-
-
Evzio
Yes
No
Yes
-
Yes
-
-
Wisconsin
Naloxone
Yes
Yes
No
-
No
-
-
Narcan
Yes
Yes
No
-
No
-
-
Evzio
No
-
-
-
-
-
-
Wyoming
Naloxone
Yes
No
-
c
-
Yes
-
-
Narcan
Yes
No
-
c
-
Yes
-
-
Evzio
Yes
No
Yes
-
Yes
-
-
Totals for All States
Yes
-
51
43
21
2
25
0
2
No
-
0
6
28
0
8
0
3
Unknown
-
2
4
4
51
20
53
48
Abbreviations: FFS, fee-for-service; MCO, managed care organization.
a
Dashes indicate unknown or not applicable for each item.
b
Materials reviewed were those available in the third quarter of 2018.
c
See state information below.
Alabama: Evzio is explicitly not covered.
Alaska: The state does not list any on the PDL, and only Evzio is identified as requiring prior authorization, quantity limits,
and step therapy. The state does reimburse for naloxone and Narcan.
Arizona: There is no evidence of coverage of Evzio.
Arkansas: Narcan has a quantity limit; it is unclear whether Evzio does. Evzio requires manual review for prior
authorization.
Colorado: Generic naloxone and Narcan are covered without prior authorization. Evzio is not covered.
Connecticut: There is no evidence that Evzio is covered. Naloxone generic and Narcan are preferred.
Florida: There is no evidence that Evzio is covered. Naloxone generic and Narcan are preferred.
Georgia: Naloxone and Narcan are preferred on the FFS PDL. Narcan and Evzio require prior authorization, and Evzio
has quantity limits.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
111
Hawaii: The FFS formulary lists only naloxone as covered; the CMS data show a minimal quantity reimbursed. The
selected MCO does not include naloxone but does include Narcan with quantity limits, and the CMS data show
reimbursement for Narcan. There is no evidence of coverage of Evzio.
Idaho: There is no evidence that Evzio is covered by the state Medicaid program.
Illinois: There is no evidence that Evzio is covered by the state Medicaid program.
Iowa: There is no evidence that Evzio is covered by the state Medicaid program.
Kansas: The CMS Medicaid Drug Utilization data for 2018 indicate the program has reimbursed for both naloxone and
Narcan. There is no evidence of reimbursement of Evzio.
Kentucky: Requirements vary greatly by MCO. None prefer Evzio, and where it is listed, it has prior authorization and
quantity limits. Some MCOs prefer naloxone and Narcan; some require prior authorization, and others do not. Similarly,
some impose quantity limits and others do not.
Louisiana: Naloxone and Narcan are preferred. At least one MCO has quantity limits.
Maine: Narcan has a quantity limit of two units per 28 days.
Massachusetts: The state apparently does not reimburse for Evzio, and the CMS 2018 Medicaid Drug Utilization data
indicate no reimbursement in this year.
Michigan: The state apparently does not reimburse for Evzio, and the CMS 2018 Medicaid Drug Utilization data indicate
no reimbursement in this year.
Minnesota: The state stopped reimbursing for Evzio in 2017.
Mississippi: FFS Medicaid covers all three, with naloxone and Narcan having preferred status and no prior authorization.
Evzio is not preferred by FFS, and at least one MCO does not cover it. The MCO imposes quantity limits on the other two
formulations.
Missouri: The state apparently does not reimburse for Evzio, and the CMS 2018 Medicaid Drug Utilization data indicate
no reimbursement in this year.
Nebraska: Evzio is expressly not covered on the formulary.
Nevada: All three are preferred on the FFS PDL. At least one new MCO identifies only naloxone and Narcan as preferred
and imposes quantity limits.
New Jersey: MCO treatment varies, but a selected MCO treats all as preferred at different tiers, requiring quantity limits
only for generic naloxone.
New Mexico: MCOs treat the three formulations differently. Two do not reimburse for Evzio, and the MCO that does
requires prior authorization and quantity limits. One requires prior authorization and quantity limits for Narcan.
New York: Evzio is not on the FFS PDL. It is identified as nonpreferred with quantity and step therapy limits on the
selected MCO formulary. The other two formulations are preferred with quantity limits for the MCO.
North Carolina: The state apparently does not reimburse for Evzio, and the CMS 2018 Medicaid Drug Utilization data
indicate no reimbursement in this year.
North Dakota: All three formulations are covered. Naloxone does not require prior authorization, and Narcan only
requires it after the first dispensing. Evzio requires prior authorization. Naloxone is covered without quantity limits; both
other formulations have quantity limits and a requirement of psychosocial treatment. Although not step therapy, the
prescriber of Evzio must justify why the other formulations are not being prescribed.
Ohio: Naloxone and Narcan are on the list of drugs for which no prior authorization is required. There also are no quantity
limits. Evzio is not covered by the FFS plan, nor was there reimbursement in the 2018 CMS data. The selected MCO only
lists the generic on the PDL.
Oklahoma: Evzio is explicitly not covered.
Oregon: The Oregon Health Plan formulary imposes quantity limits on naloxone and Narcan. Evzio was not reimbursed in
2018.
Pennsylvania: Evzio is reimbursed but is not preferred and requires prior authorization.
Puerto Rico: There is no evidence of coverage.
Rhode Island: The state does not cover Evzio. A selected MCO requires quantity limits for naloxone and Narcan.
South Carolina: FFS covers naloxone and Narcan as preferred. It expressly does not cover Evzio. The selected MCO
covers all three formulations with quantity limits, treating Evzio as nonpreferred and requiring step therapy and prior
authorization.
South Dakota: All are covered. Evzio requires prior authorization.
Tennessee: Narcan is preferred, requires prior authorization, and has a quantity limit. Naloxone is reimbursed. Evzio is
not.
Texas: There is no evidence of coverage of Evzio.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
112
Utah: Evzio is not covered. The FFS PDL and other documents do not include any formulation, leaving requirements
uncertain. The selected MCO covers naloxone and Narcan without limit.
Vermont: Narcan has a quantity limit, and Evzio is not covered.
Washington: There is no evidence of coverage of Evzio.
West Virginia: All three are covered, with naloxone and Narcan preferred with no prior approval. The drug limits
document indicates that quantity limits apply for generic naloxone and Evzio. The drug code document indicates that
quantity limits apply for injection naloxone (with Narcan listed as the brand alternative).
Wisconsin: Evzio is not covered.
Wyoming: Any Evzio requires prior authorization. The other two formulations require prior authorization after the first fill.
MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
113
VIII. Appendix B. Authors and Acknowledgements
Authors
Peggy O’Brien
Shums Alikhan
Nicholas Cummings
Andriana Hohlbauch
Lauren Hughey
Kristin Schrader
Ali Bonakdar Tehrani
Mackenzie White
Acknowledgements
The authors would like to thank the interviewees and organizations who assisted in the
preparation of this report, as well as Mitchell Berger and Sarah Steverman at SAMHSA. We
also wish to acknowledge Mustafa Karakus, Norah Mulvaney-Day, Paige Jackson, Azra Jaferi,
Lucy Karnell, Linda Lee, and Alex Waddell at IBM® Watson Health™ and Megan Dormond at
the National Council for Behavioral Health for their assistance.

MEDICAID COVERAGE OF MEDICATION-ASSISTED TREATMENT FOR ALCOHOL AND OPIOID USE
DISORDERS AND OF MEDICATION FOR THE REVERSAL OF OPIOID OVERDOSE
114
HHS Publication No. SMA-18-5093
Published 2018
U.S. Department of Health and Human Services
Substance Abuse and Mental Health Services Administration
SAMHSA’s mission is to reduce the impact of substance abuse and mental illness on
America’s communities.
1-877-SAMHSA-7 (1-877-726-4727) • 1-800-486-4889 (TDD) • www.samhsa.gov
